Customer Feedback for GS441524
Feline Infectious Peritonitis (FIP) is a fatal viral disease caused by specific strains of the feline coronavirus (FCoV), known as feline infectious peritonitis virus (FIPV). Before the discovery of GS441524, FIP was considered incurable, with a mortality rate of nearly 100%. Affected cats typically suffered from severe symptoms such as fever, weight loss, fluid accumulation in the abdomen or chest, and neurological issues, leading to rapid deterioration and death. GS441524 has revolutionized FIP treatment, offering the first effective therapeutic option for this devastating disease.
First-time purchase



Feedbacks
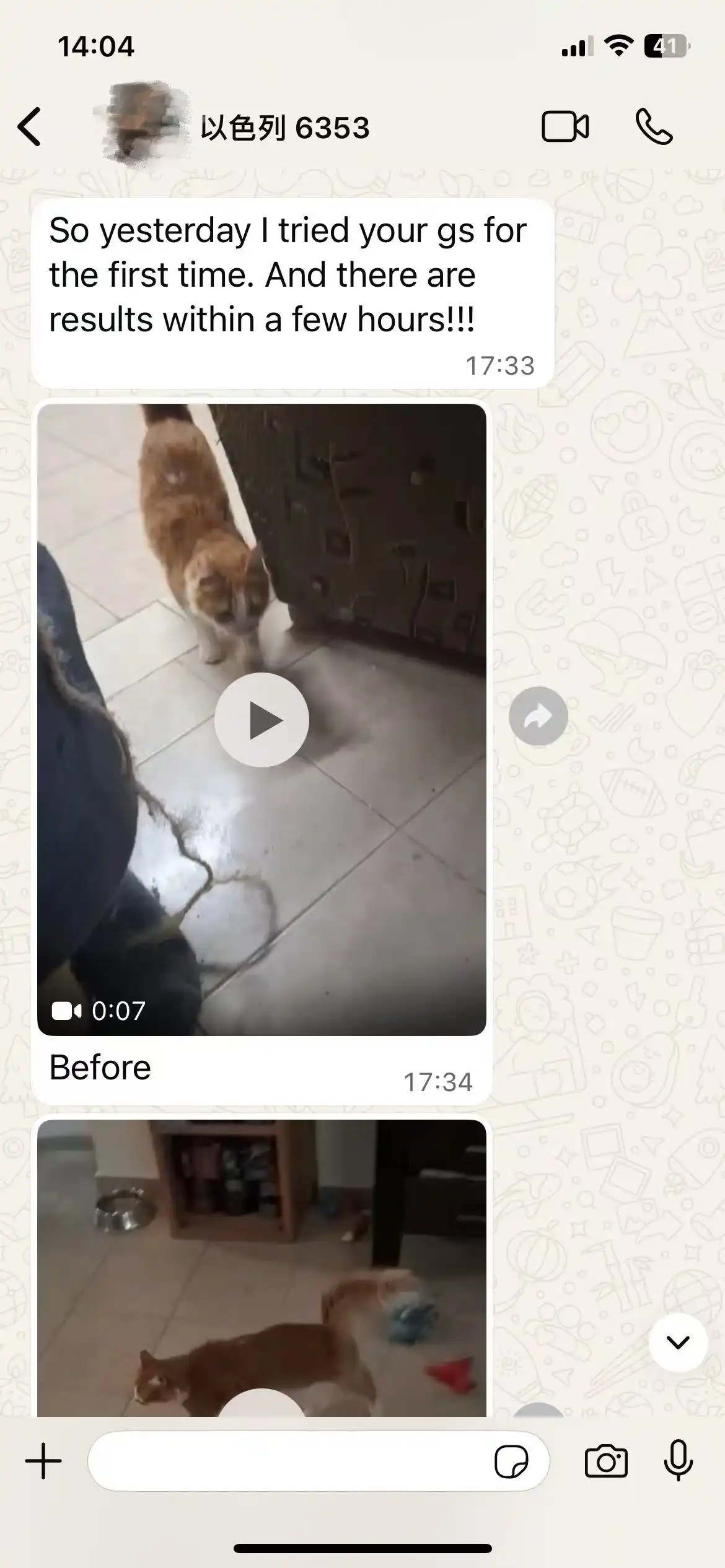
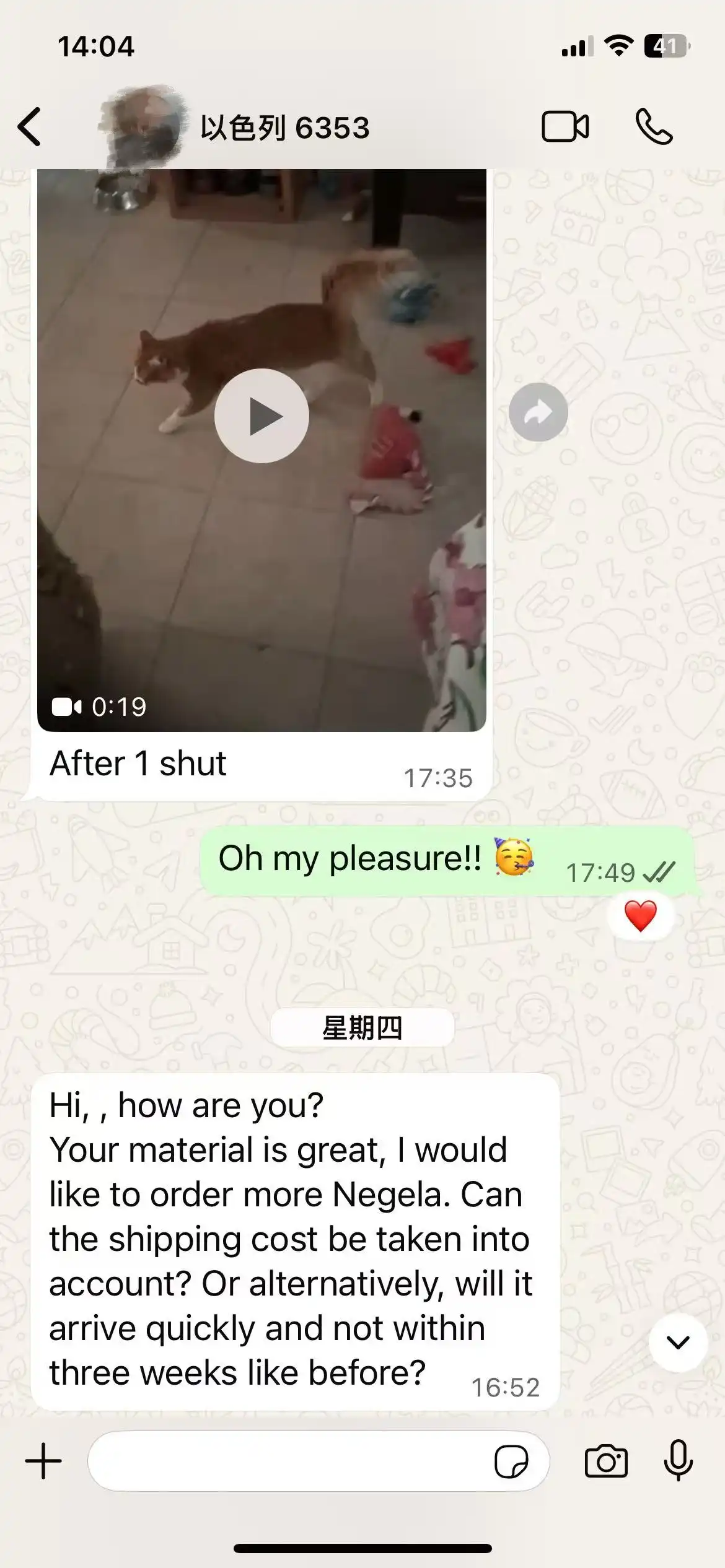
Frequently Asked Questions from Customers
Q1:What is your shipping methods?
A2:Shipping process:
We clear customs for you and set up the warehouse in your local place.
(1)4-8 days
We established a company in your local place, this company is the buyer, the normal international trading between this two companies, complete documents will pass the customs.
(2)0-2 days
After clear the goods, pick up from warehouse or deliver to you.
For you, absolutely! No buying record, No signature, storing for you with warehouse!
Q2:How do i pay?
A2:Payment methods:
(1)T/T (Bank transfer)
Now we only can receive USD only.
2-3 days for funds to be credited.
(2)Bitcoin or USDT
Convenient & fast for the individual clients.
Q3:Does it come with a maunfacturing date?
A3:Has a production date. And the shelf life of three years.
Q4:My cat has been diagnosed with FIP. What is the success rate of treatment with GS441524?
A4:Based on clinical studies and real-world case data, the overall success rate of GS441524 in treating FIP is approximately 80%-85%.
Q5:How should I store the purchased GS441524?
A5:Store refrigerated at 2–8°C.
Reducing the Risk of Recurrence

Follow-up Protocol: Phased Monitoring of Viral Load and Organ Function
At Treatment Completion (Weeks 12/16/20): Complete the “Blood Test + Viral Detection” package, including:
- Complete Blood Count (CBC) to assess inflammation resolution
- Biochemical Panel (liver/kidney function, albumin-globulin ratio)
- FIPV RNA Detection (blood + feces) to screen for residual virus. Clinical cure is confirmed if the W/S ratio returns to ≥0.8 and FIPV RNA is negative.
1 month post-treatment: Re-test FIPV RNA (focusing on blood) to confirm no viral rebound. If the cat previously exhibited neurological symptoms, additional brain imaging (e.g., MRI) is required to rule out residual granulomas.
3-6 months post-treatment: Conduct comprehensive physical examination (including abdominal ultrasound to assess liver/spleen size normalization). If all parameters are normal, subsequent monitoring may follow standard feline intervals (annual check-ups) without frequent follow-ups.

Long-term Care

Diet Management
Continue feeding high-protein food for 1-2 months post-recovery, then gradually transition to regular cat food to avoid gastrointestinal upset from sudden dietary changes. Avoid raw meat and eggs (to prevent Salmonella infection; immunity remains compromised for 1 year after FIP recovery).
Vaccinations
Administer core vaccines (feline distemper, feline calicivirus, feline parvovirus) 1-2 months post-treatment. Do not administer FIP vaccines (no effective FIP vaccine currently exists). Test antibody levels before vaccination to ensure boosts are given only when immunity is insufficient, avoiding unnecessary immune system stimulation.


Environmental Adjustment
Recovered cats may gradually resume normal activities, but strenuous exercise (e.g., climbing heights, prolonged chasing) should be avoided. This is especially critical for cats with a history of pleural effusion, as full pulmonary function recovery typically takes 3-6 months.
The application of GS441524 in treating feline infectious peritonitis (FIP) has achieved a breakthrough from “untreatable” to “high cure rates.” Its mechanism of targeting and inhibiting RNA viruses also offers new insights for treating other RNA viral infections in cats. Currently, development of an oral formulation of GS441524 for cats has progressed (e.g., the microcapsule oral formulation launched in 2024), achieving 35% oral bioavailability. This formulation can replace subcutaneous injections, addressing the issue of stress-induced resistance in cats, and is expected to become the mainstream clinical administration method in the future. Concurrently, studies on combination therapies (e.g., with interferon to enhance immunity) and dose optimization for drug-resistant FIPV variants are advancing. As research deepens, GS441524 is poised to play an increasingly vital role in feline healthcare, offering renewed hope for recovery to more affected cats.
Safety Profile

The safety profile of GS-441524 has been extensively evaluated in both preclinical and clinical studies. In general, the compound is well-tolerated by cats and humans, with a low incidence of adverse effects. The most common side effects observed in cats treated with GS-441524 for FIP include mild gastrointestinal disturbances, such as vomiting and diarrhea, which are usually transient and resolve on their own.
In human studies, the safety of Remdesivir (which contains GS-441524 as its active metabolite) has been evaluated in clinical trials involving patients with COVID-19. The results showed that Remdesivir was generally well-tolerated, with the most common adverse effects being nausea, vomiting, and elevated liver enzymes. However, these side effects were usually mild to moderate in severity and did not require discontinuation of treatment.
It is important to note that the safety of GS-441524 has not been fully evaluated in all populations, particularly in pregnant women, children, and individuals with underlying medical conditions. Therefore, caution should be exercised when using GS-441524 in these vulnerable groups, and further research is needed to determine its safety and efficacy in these populations.


Abby
7 years of experience in chemical articles; Master's degree; Organic Chemistry major; R&D-4 Dept; Technology support; R&D engineer
Anticipating your Business & Technology support inquiry
Please send us the products that interest you, and we will provide you with one-on-one service
Recommended Blog

GS-441524 Injection vs. Tablets: Which Form is Better for Your Cat?
How to Create a GS-441524 Treatment Plan After an FIP Diagnosis?

GS-441524 Demystified: A Complete Guide to Its Mechanism, Efficacy, and Safety
From Despair to Hope: A Cat Owner's GS-441524 Treatment Journey

The Most Important Nursing Care Tips for Cats on GS-441524

How to Tell if Your Cat is Fully Cured After GS-441524 Treatment?
Top Reasons Why GS-441524 Treatment Fails: Common Mistakes to Avoid

Does My Cat Need a Special Diet During GS-441524 Treatment?