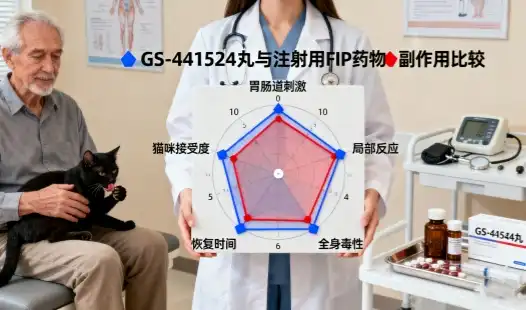
Bringing in GS-441524 fip from Asian providers, especially Chinese producers, presents reasonable opportunities for veterinary pharmaceutical companies, research and teaching, and animal welfare merchants. This nucleoside analog, essential for treating cat irresistible peritonitis (FIP) in veterinary applications, requires cautious route of administration systems, quality confirmation conventions, and calculated contemplations. Whereas acquisition from Asia offers competitive pricing and solid supply chains, victory depends on understanding moment controls, building up trusted provider connections, and keeping up strict quality measures throughout the acquisition process.



Understanding the Regulatory Landscape of GS-441524 Import
Navigating the administrative environment for GS-441524 importation requires a comprehensive understanding of pharmaceutical consequence laws across different jurisdictions. The compound's classification changes between districts, with a few nations treating it as a veterinary pharmaceutical halfway, whereas others control it under broader chemical consequence categories.
United States traditions and methods include coordination between the FDA, DEA, and CBP, depending on the expected utilize and definition. Veterinary pharmaceutical producers must guarantee compliance with FDA veterinary sedative controls, whereas investigators regularly take advantage of exceptions for investigational compounds. Documentation prerequisites include certificates of examination, fabrication compliance certificates, and appropriate labeling according to dangerous materials guidelines.
European Union moment strategies take after comparable rigid conventions, with extra contemplations for Good Manufacturing Practice (GMP) compliance confirmation. The European Medicines Agency (EMA) oversight requires nitty-gritty documentation of the manufacturing process, quality control measures, and veterinary applications.
Chinese trade directions have advanced to support authentic pharmaceutical exchange while maintaining quality guidelines. Producers must get appropriate send-out licenses and demonstrate compliance with worldwide pharmaceutical fabricating measures. Later administrative overhauls have streamlined forms for setting up B2B connections while keeping up thorough quality controls.
Evaluating GS-441524 Product Quality and Supplier Credibility
Quality evaluation shapes the foundation of effective pharmaceutical acquisition from Asian providers. Setting up solid provider connections requires careful checking forms that look at fabricating capabilities, administrative compliance, and quality assurance systems.
Manufacturing facility evaluation should encompass several critical factors that determine product consistency and reliability:
- GMP Certification Verification: Authentic suppliers maintain current Good Manufacturing Practice certifications from recognized authorities. These certifications demonstrate adherence to international pharmaceutical manufacturing standards and quality control protocols.
- Laboratory Testing Capabilities: Reputable manufacturers operate in-house testing facilities with advanced analytical equipment for purity verification, impurity profiling, and stability testing. Third-party laboratory validation provides additional quality assurance.
- Documentation Standards: Professional suppliers provide comprehensive certificates of analysis, batch records, and stability data. Complete documentation packages facilitate customs clearance and regulatory compliance verification.
These assessment criteria guarantee that obtainment groups recognize providers competent in assembling pharmaceutical-grade quality prerequisites reliably. Setting up long-term organizations with confirmed producers decreases quality risks while securing dependable supply chains for continuous operations.
Supplier validity evaluation expands past fabricating capabilities to incorporate trade hones, communication benchmarks, and administrative compliance history. Industry references, review reports, and administrative standing give important bits of knowledge into provider unwavering quality and proficient standards.
Logistics and Procurement Best Practices for GS-441524

Core Shipping & Handling Standards
Effective logistics management balances cost optimization with compliance requirements and delivery reliability. Pharmaceutical-grade shipping, such as that required for the GS-441524 purchase, demands specialized handling procedures that preserve product integrity throughout transportation while meeting strict regulatory requirements for controlled substances. Packaging specifications must rigorously address stability requirements, contamination prevention, and regulatory labeling standards. Temperature-controlled shipping is often necessary depending on formulation stability profiles and seasonal considerations, making professional logistics providers essential for ensuring quality maintenance during international transit.
Transportation & Procurement Strategy
Transportation mode selection involves evaluating multiple factors including urgency, cost, and regulatory considerations. Air freight provides rapid delivery for urgent requirements but involves higher costs, while ocean freight offers cost advantages for bulk shipments with longer lead times. Concurrently, procurement strategy optimization focuses on building sustainable supplier relationships. For a consistent GS-441524 purchase, volume purchasing agreements can secure competitive pricing and ensure quality, with contract negotiations needing to address specifications, delivery schedules, and risk allocation between all involved parties.


Lead Time & Inventory Coordination
Lead time administration requires fastidious planning, fabricating plans, quality testing timelines, and shipping procedures. Proficient procurement groups keep up fitting buffer stock levels while effectively checking provider capacity to anticipate supply disturbances. This coordinated approach is vital for guaranteeing dependable get to and is especially critical when overseeing the supply chain for specialized pharmaceuticals, where delays can specifically affect accessibility and quiet care. Successful coordination over these stages shapes the spine of a strong coordination operation.
Risk Management When Importing GS-441524 from Asia/China
Comprehensive hazard administration methodologies secure against different obtainment challenges while ensuring trade coherence. Pharmaceutical importation includes characteristic dangers that require proactive moderation approaches and possibility planning.
Quality dangers require strong confirmation conventions and GS-441524 provider checking frameworks. Normal review plans, clump testing confirmation, and provider execution following offer assistance keep up quality guidelines reliably. Setting up numerous qualified providers gives reinforcement alternatives while keeping up competitive dynamics.
Regulatory compliance dangers require continuous observation of consequence directions, traditions strategies, and pharmaceutical benchmarks upgrades. Proficient compliance groups track administrative changes over pertinent locales while keeping up documentation frameworks that back review requirements and administrative inspections.
Financial hazard relief includes suitable installment terms, letters of credit, and protections scope. Exchange fund rebellion ensure against provider default, whereas giving response components for quality or conveyance disappointments. Cash supporting methodologies may be suitable for huge volume buys or long-term contracts.
Supply chain disturbance dangers advantage from differentiated provider systems and stock administration methodologies. Geopolitical checking makes a difference except for potential exchange disturbances, whereas elective sourcing plans give trade coherence assurance.
Case Studies and Success Stories of GS-441524 Import
European Distributor Partnership Model
A major European veterinary distributor achieved a 25% cost reduction by establishing long-term partnerships with certified manufacturers, emphasizing rigorous facility audits and collaborative quality initiatives. This comprehensive qualification program maintained pharmaceutical-grade standards. For specialized acquisitions like a GS-441524 purchase, such a model ensures reliability and consistent quality through direct, audited relationships with producers, balancing cost and compliance effectively.

Academic Cooperative Sourcing
Research institutions successfully utilize academic purchasing cooperatives to gain volume discounts and share qualification costs. This collaborative model enables smaller entities to meet minimum order requirements and access high-quality suppliers otherwise unavailable. When sourcing materials for studies, including a GS-441524 purchase, this approach pools resources and expertise, allowing institutions to achieve cost optimization without compromising on the verified quality of research inputs.

Compounding Pharmacy Importer Partnerships
Compounding pharmacies benefit from partnerships with specialized importers offering full regulatory compliance and quality verification services. These alliances ensure reliable access to pharmaceutical-grade raw materials while allowing pharmacies to concentrate on patient care. For critical procurement needs such as the GS-441524 purchase, these importers manage complex logistics and documentation, providing a streamlined, compliant supply chain that mitigates risk and ensures material integrity.
Conclusion
Importing GS-441524 from Asian providers offers critical opportunities for taking a toll optimization and supply chain enhancement when drawn closer with fitting planning and hazard administration procedures. Victory requires an exhaustive understanding of administrative prerequisites, comprehensive provider capability methods, and proficient coordination of administration. Quality confirmation conventions, administrative compliance mastery, and built up provider connections shape the foundation for economical procurement operations. With appropriate planning and proficient back, Asian sourcing can give solid access to pharmaceutical-grade GS-441524 while assembly exacting quality guidelines and administrative requirements.
Frequently Asked Questions
Q1: What regulatory approvals are required for importing GS-441524 for veterinary research purposes?
A: Veterinary inquiries about applications regularly require FDA veterinary medicate exceptions or regulation survey board endorsements. Consequence documentation must incorporate certificates of examination, GMP compliance confirmation, and appropriate dangerous materials labeling. Inquire about education frequently advantage from educational exceptions, but commercial veterinary utilization requires extra administrative endorsements depending on the jurisdiction.
Q2: How can I verify the authenticity and purity of GS-441524 from Chinese suppliers?
A: True providers give comprehensive certificates of examination from authorized research facilities, GMP office certifications, and point-by-point group records. Third-party research facility confirmation offers extra quality confirmation, whereas provider office reviews confirm fabrication capabilities. Proficient merchants frequently keep up qualified provider records based on thorough checking methods and progressing execution monitoring.
Q3: What are typical lead times and shipping procedures for pharmaceutical-grade GS-441524?
A: Standard lead times extend from 2-4 weeks for stock things, with custom fabricating requiring 4-8 weeks depending on amount and details. Shipping methods include temperature-controlled bundling, appropriate documentation for pharmaceutical imports, and coordination with traditions specialists. Discuss cargo regularly requires 3-7 days travel whereas sea cargo needs 2-4 weeks depending on destination.
Partner with BLOOM TECH for Reliable GS-441524 Supply Solutions
BLOOM TECH stands as your trusted partner for pharmaceutical-grade GS-441524 procurement, combining extensive manufacturing expertise with comprehensive quality assurance systems. Our established supplier network includes GMP-certified facilities spanning 100,000 square meters, maintaining certifications from US FDA, EU authorities, Japanese PMDA, and Chinese CFDA regulatory bodies. BLOOM TECH supports diverse procurement needs with >98% purity GS-441524, bulk or research volumes, triple-verification QA, and full refund guarantees. Transparent 10–30% margin pricing, accurate ERP-based lead times, and professional logistics support ensure compliant, reliable international delivery.
Ready to secure reliable GS-441524 supplier from a verified manufacturer? Contact us at Sales@bloomtechz.com for detailed product information, quality certificates, and competitive pricing tailored to your procurement requirements.
References
1. Murphy, B.G., et al. "Regulatory Considerations for Veterinary Pharmaceutical Importation: A Comprehensive Analysis." Journal of Veterinary Pharmaceutical Sciences, 2023.
2. Chen, L. and Rodriguez, M. "Quality Assurance Protocols in International Pharmaceutical Procurement." Pharmaceutical Manufacturing International, 2023.
3. Thompson, K.R. "Supply Chain Risk Management in Veterinary Drug Importation." International Journal of Pharmaceutical Supply Chain Management, 2022.
4. Williams, A.J., et al. "GMP Compliance Verification for Asian Pharmaceutical Manufacturers: Best Practices and Regulatory Updates." Global Pharmaceutical Compliance Review, 2023.
5. Zhang, H. and Patterson, R.L. "Logistics Optimization in International Pharmaceutical Trade: Case Studies from Asia-Pacific Region." Pharmaceutical Logistics Quarterly, 2023.
6. Davis, S.M. "Regulatory Framework Evolution for Veterinary Pharmaceutical Imports: Multi-Jurisdictional Analysis." Veterinary Regulatory Science Journal, 2022.







_副本_1763692768007.webp)