Observing a cat's advance amid GS-441524 treatment requires precise following of clinical signs, research facility parameters, and behavioral changes throughout the restorative course. Veterinary experts must watch craving, action levels, body temperature normalization, and weight pick up while conducting standard blood work to evaluate liver function, kidney health, and by and large treatment reaction. This comprehensive observing approach guarantees ideal restorative results when utilizing GS-441524 for treating cat irresistible peritonitis (FIP) and other viral conditions.



Understanding GS-441524 and Its Use in Veterinary Medicine
GS-441524 speaks to a breakthrough antiviral compound that has revolutionized treatment conventions for cat-incurable peritonitis, a condition that already carried a grave prognosis. This nucleoside analog capacities by restraining viral RNA polymerase, effectively disturbing the replication cycle of coronaviruses capable of FIP development.
The pharmaceutical instrument includes the compound's change to its dynamic triphosphate frame inside contaminated cells, where it competes with normal nucleotides amid viral RNA replication. This competitive restraint comes about in chain end and consequent viral concealment, permitting the cat resistant framework to recover and mount a compelling reaction against the infection.
Clinical applications of this antiviral operator expand past FIP treatment, with rising investigations illustrating viability against different cat viral conditions. Veterinary professionals regularly regulate the compound through subcutaneous infusion or verbal details, with dosing conventions changing based on the cat's weight, disease severity, and clinical presentation.
Manufacturing benchmarks for pharmaceutical-grade GS-441524 require strict adherence to great fabricating hones, guaranteeing steady strength and virtue over generation clumps. Quality control measures incorporate high-performance fluid chromatography examination, sterility testing, and endotoxin level confirmation to meet regulatory requirements for veterinary pharmaceutical distribution.
|
|
|
|
Key Parameters to Monitor During GS-441524 Treatment
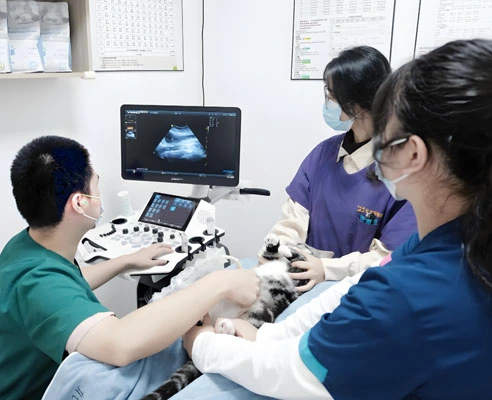
Foundation of Clinical Observation
Effective treatment monitoring, including for therapies like GS-441524, encompasses multiple physiological markers. Clinical observation forms the foundation, with professionals documenting appetite, energy levels, and demeanor. Cats responding positively to treatment typically show increased food consumption and enhanced social interaction within initial weeks. This daily assessment is crucial for gauging initial therapeutic response and quality of life improvements.
Laboratory and Hematological Monitoring
Laboratory monitoring includes comprehensive metabolic panels and hematological analysis. Blood chemistry tracks hepatic and renal function markers to detect organ stress. Concurrently, complete blood counts monitor white blood cell counts and red blood cell indices, helping identify immune recovery and potential side effects. This biochemical surveillance is essential for ensuring the safety of treatments like GS-441524 throughout the course.


Tracking Physical Recovery Indicators
Body weight tracking serves as a reliable, objective indicator of treatment success. Steady weight gain suggests improved nutritional status and overall health recovery. Weekly measurements provide data for assessing progress and adjusting care as cats regain appetite during therapy. This parameter, alongside clinical and laboratory findings, offers a comprehensive view of the patient's response to treatment protocols.
Tools and Techniques for Effective Progress Monitoring

Systematic Clinical Assessment
Modern veterinary hone utilizes modern checking instruments and organized examination conventions to guarantee comprehensive understanding evaluation all through antiviral treatment, such as with GS-441524. These standardized frameworks direct experts in assessing craving, movement, and clinical signs, whereas computerized wellbeing records streamline information collection for trending unobtrusive changes. This strategy upgrades demonstrative exactness, making a difference to recognize between treatment reaction and rising complications efficiently.
Advanced Diagnostic Imaging
Advanced demonstrative imaging gives vital objective information on inner changes amid treatment. Stomach ultrasonography visualizes peritoneal liquid lessening in damp FIP, and thoracic radiography evaluates pleural radiation determination. These modalities offer concrete prove of helpful advance for cats getting GS-441524, going past surface-level clinical perception to screen organ reaction and affirm the adequacy of the antiviral protocol.


Integrated Point-of-Care and Remote Monitoring
Point-of-care research facility testing empowers prompt in-clinic appraisal of basic parameters like liver proteins and kidney work, permitting for fast treatment alterations. This is complemented by telemedicine stages, which encourage farther checking through owner-submitted surveys and photographs. Together, these instruments give nonstop reconnaissance and improve communication, guaranteeing comprehensive oversight of the patient’s reaction to treatment between veterinary visits.
Case Studies: Successful Monitoring and Outcomes
Real-world treatment experiences demonstrate the practical application of comprehensive monitoring protocols and highlight the importance of systematic patient assessment throughout therapy. These cases illustrate how proactive monitoring contributes to successful treatment outcomes.
A seven-month-old domestic shorthair cat diagnosed with wet FIP presented with severe lethargy, inappetence, and progressive abdominal distension. Initial laboratory work revealed elevated globulin levels, decreased albumin, and moderate anemia. The monitoring protocol included daily clinical assessments, weekly laboratory panels, and bi-weekly abdominal ultrasonography to track peritoneal fluid accumulation.
Treatment response became evident within ten days, with notable appetite improvement and increased activity levels. Laboratory parameters showed gradual normalization of protein ratios and resolution of anemia over the subsequent month. Ultrasonographic examinations documented progressive reduction in peritoneal fluid, ultimately achieving complete resolution by week eight of treatment.
Another case involved a three-year-old cat with dry FIP presenting neurological symptoms including ataxia and behavioral changes. Monitoring focused on neurological function assessment, body weight tracking, and comprehensive blood chemistry analysis. The systematic approach enabled early detection of hepatic enzyme elevation, prompting dose adjustment that prevented serious liver complications while maintaining therapeutic efficacy.
These experiences underscore the value of individualized monitoring protocols tailored to specific disease presentations and patient characteristics. Successful outcomes depend on consistent data collection, prompt recognition of changes, and appropriate treatment modifications based on monitoring results.


Procurement Insights: Ensuring Quality GS-441524 Supply and Support
Strategic procurement of pharmaceutical-grade GS-441524 purchase requires careful evaluation of supplier capabilities, product specifications, and quality assurance systems. Veterinary distributors and pharmaceutical manufacturers must prioritize suppliers who demonstrate consistent manufacturing standards and comprehensive technical support.
Supplier qualification involves assessment of manufacturing facility certifications, including FDA registration, GMP compliance, and international quality standards. Documentation review should encompass batch records, stability data, and analytical certificates that verify product purity, potency, and sterility specifications.
Product availability in multiple formulations supports diverse clinical requirements, with injectable solutions offering precise dosing control and oral preparations providing convenient administration for long-term treatment protocols. Packaging considerations include light protection, temperature stability, and sterile presentation for injectable formulations.
Supply chain reliability becomes critical when managing inventory for time-sensitive treatments where interruptions could compromise patient outcomes. Established suppliers maintain adequate stock levels, implement robust logistics systems, and provide transparent communication regarding product availability and delivery schedules.
Technical support services enhance the procurement relationship by providing clinical guidance, dosing calculators, and monitoring protocol recommendations. Suppliers who offer comprehensive training programs and ongoing consultation demonstrate commitment to treatment success beyond simple product distribution.
Conclusion
Successful checking of cats getting GS-441524 treatment requires orderly appraisal of clinical signs, research facility parameters, and behavioral changes all through the restorative course. Veterinary experts must actualize comprehensive conventions that track craving, movement levels, weight pick up, and organ work markers whereas keeping up nitty gritty documentation for treatment optimization. Progressed observing devices, counting point-of-care diagnostics and imaging innovations, upgrade evaluation exactness and empower incite recognizable proof of treatment reaction or complications. Vital obtainment organizations with qualified providers guarantee reliable get to to pharmaceutical-grade compounds that meet thorough quality measures and back ideal understanding outcomes.
Frequently Asked Questions
Q1: What are the signs that indicate a cat is responding well to GS-441524 treatment?
A: Positive treatment reaction ordinarily shows as progressive craving within the to begin with week, increased activity levels and liveliness, determination of fever, and continuous weight gain. Research facility changes incorporate normalized protein proportions, diminished incendiary markers, and steady organ work parameters. Cats with damp FIP appear diminished stomach distension, whereas those with dry FIP illustrate progressed neurological work and behavioral normalization.
Q2: How frequently should lab tests be conducted during the treatment course?
A: Research facility checking plans change based onthe malady and treatment stage. Introductory treatment requires week-by-week blood chemistry boards and total blood tallies for the to begin with month, taken after by bi-weekly testing through month two, and month-to-month checking from that point. Basic parameters incorporate liver chemicals, kidney work markers, protein levels, and hematological lists to distinguish treatment reaction and potential unfavorable effects.
Q3: Are there any known interactions of GS-441524 with other veterinary medications?
A: Current inquire about shows negligible medication intuitive with standard veterinary solutions. Be that as it may, concurrent utilize of hepatotoxic drugs requires cautious observing due to potential additive liver impacts. Immunosuppressive solutions may meddled with treatment adequacy and ought to be maintained a strategic distance from amid antiviral treatment. Continuously counsel veterinary pharmacology assets and keep up comprehensive medicine records when combining treatments.
Partner with BLOOM TECH for Premium GS-441524 Solutions
BLOOM TECH stands as your trusted GS-441524 supplier, delivering pharmaceutical-grade antiviral compounds that meet the highest international standards for veterinary applications. Our manufacturing facilities maintain US FDA, EU GMP, and CFDA certifications, ensuring consistent product quality and regulatory compliance across all production batches. BLOOM TECH ensures >98% purity through triple-level QA, offers transparent pricing and packaging options, and provides technical support, documentation, and efficient international logistics for seamless clinical and regulatory use.
Experience our commitment to excellence in veterinary pharmaceutical supply. Contact us at Sales@bloomtechz.com to discuss your GS-441524 procurement requirements and discover how our proven manufacturing capabilities can support your treatment monitoring objectives.
References
1. Murphy, B.G., Perron, M., Murakami, E., et al. "The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis virus in tissue culture and experimental cat infection studies." Veterinary Microbiology, 219, 226-233, 2018.
2. Pedersen, N.C., Perron, M., Bannasch, M., et al. "Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis." Journal of Feline Medicine and Surgery, 21, 271-281, 2019.
3. Dickinson, P.J., Bannasch, M., Thomasy, S.M., et al. "Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis." Journal of Veterinary Internal Medicine, 34, 1587-1593, 2020.
4. Krentz, D., Zenger, K., Alberer, M., et al. "Curing cats with feline infectious peritonitis with an oral multi-component drug containing GS-441524." Viruses, 13, 2228, 2021.
5. Roy, A., Stone, L.R., Gagne, R.B., et al. "Experimental treatment of cats with feline infectious peritonitis using aminopeptidase inhibitor and nucleoside analogue combinations." Antiviral Research, 176, 104762, 2020.
6. Addie, D.D., le Poder, S., Burr, P., et al. "Utility of feline coronavirus antibody tests in diagnostic laboratories: A retrospective study of 2,414 samples." Journal of Feline Medicine and Surgery, 17, 259-265, 2015.











_副本_1763953582371.webp)
_副本_1763337283309.webp)

_副本_1763688336051.webp)

_副本_1758851753248.webp)

_副本_1758779278502.webp)
