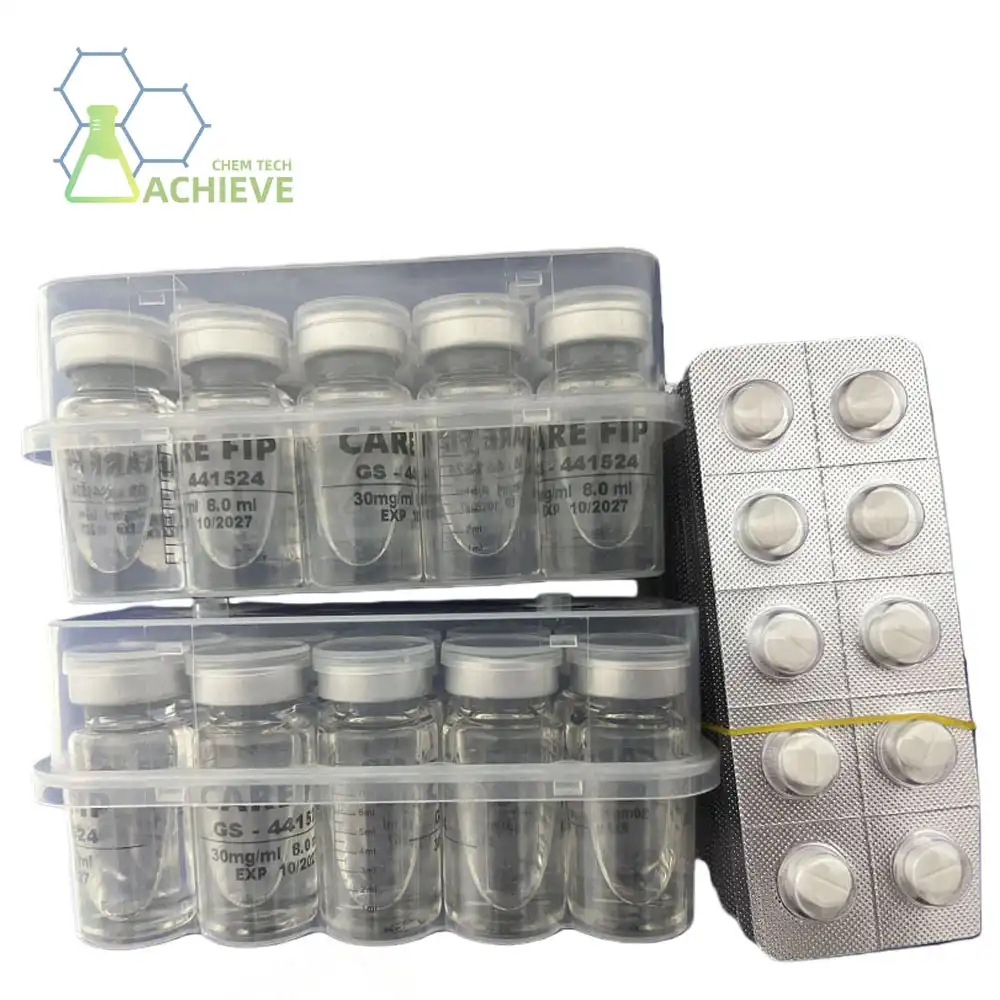
Malaysia's rising biotech division presents huge opportunities for companies specializing in antiviral therapeutics, especially those including GS-441524. This nucleoside analog has picked up noteworthy consideration for its efficacy in treating cat infectious peritonitis (FIP) and its potential applications in broader antiviral research. Malaysian biotech firms can use GS-441524's demonstrated restorative benefits to create inventive arrangements for veterinary pharmaceuticals while investigating opportunities in pharmaceutical research and development. The compound's well-documented security profile and viability make it an appealing choice for biotech companies looking to set themselves up in the competitive Asian advertising landscape.


Understanding GS-441524 and Its Mechanism of Action
The therapeutic landscape of antiviral compounds has evolved significantly, with GS-441524 emerging as a pivotal player in veterinary medicine. This nucleoside analog demonstrates remarkable antiviral properties through its unique molecular structure and targeted mechanism of action.
What is GS-441524?
GS-441524 speaks to a breakthrough in antiviral treatment, working as an adenosine nucleoside analog with demonstrated viability against coronaviruses. The compound's chemical structure permits it to meddled with viral replication forms, making it especially successful against cat coronavirus transformations that cause FIP. Pharmaceutical producers have broadly examined its pharmacological properties, uncovering amazing bioavailability and tissue infiltration characteristics that upgrade helpful outcomes.
Current endorsed applications center essentially on veterinary pharmaceutical, despite the fact that inquire about teach proceed investigating broader helpful conceivable outcomes. The compound's steadiness and solvency profile make it appropriate for different definition approaches, from injectable arrangements to verbal preparations.
Mechanism of Action and Key Benefits
The molecular action of GS-441524 centers on its ability to disrupt viral RNA synthesis by mimicking natural adenosine substrates. Once inside infected cells, the compound undergoes phosphorylation to its active triphosphate form, which then competes with natural nucleotides during viral RNA replication. This competition results in chain termination and effectively halts viral proliferation.
Clinical evidence demonstrates several compelling advantages of this antiviral approach. Veterinary practitioners have observed rapid improvement in FIP-affected cats, with many achieving complete remission when treated according to established protocols. The compound's safety profile shows minimal adverse effects when administered correctly, making it a preferred choice for long-term treatment regimens.
Research Studies and Clinical Evidence
Extensive inquire about approves the helpful potential of this nucleoside analog over numerous ponder populaces. Free veterinary trials have reliably appeared reaction rates surpassing 80% in FIP cases, with neurological and visual shapes reacting especially well to treatment conventions. Comparative ponders show prevalent results compared to strong care alone, building up clear prove for helpful intervention.
Research educate have archived comprehensive pharmacokinetic profiles, illustrating unsurprising retention and dissemination designs that bolster standardized dosing rules. These ponders shape the establishment for administrative entries and quality control measures fundamental for pharmaceutical development.
|
|
|
|
GS-441524 Use Cases Tailored to Malaysia's Biotech Firms
Malaysian biotech companies work inside a energetic biological system that offers interesting points of interest for creating and commercializing antiviral therapeutics. The region's key area, developing investigate framework, and steady administrative environment make openings for inventive applications of demonstrated antiviral compounds.
Veterinary Applications with Focus on FIP Treatment
GS-441524 has transformed FIP treatment in Malaysia, with 12-week weight-based protocols delivered subcutaneously and monitored through clinical evaluations. Early diagnosis, education, and supportive diagnostics improve outcomes across both wet and dry FIP presentations.
Potential Expansion into Human Therapeutics and Research
Malaysian biotech firms can explore GS-441524 related antiviral mechanisms through research partnerships, advancing formulation, delivery systems, and combination therapies while strengthening local scientific capabilities for broader therapeutic development.
Case Studies from Malaysian and Regional Biotech Companies
Pilot programs demonstrate effective antiviral development through collaboration with veterinary hospitals and research centers, emphasizing regulatory compliance, quality control, and thorough documentation to accelerate market acceptance and credibility.
Procurement Considerations for GS-441524 in Malaysia
Navigating the procurement landscape for pharmaceutical compounds requires careful attention to quality standards, regulatory compliance, and supplier reliability. Malaysian biotech firms must evaluate multiple factors when selecting suppliers and establishing sustainable supply chains for their operations.
How to Source Authentic GS-441524 Materials?
Supplier verification represents the most critical aspect of procurement strategy, requiring thorough evaluation of manufacturing capabilities, quality certifications, and regulatory compliance records. Reputable suppliers maintain comprehensive documentation including certificates of analysis, stability data, and manufacturing batch records that demonstrate consistent product quality.
Malaysian import regulations require proper documentation and compliance with local pharmaceutical standards. Companies must ensure suppliers can provide necessary regulatory support and maintain appropriate export licenses for international shipments.
Pricing, Bulk Orders, and Shipping Logistics
Market pricing reflects various factors including purity levels, packaging specifications, and order quantities. Bulk purchasing often provides significant cost advantages while ensuring consistent supply availability for ongoing operations. Effective logistics management requires coordination with reliable shipping partners experienced in pharmaceutical transport requirements.
Temperature-controlled shipping and proper handling protocols preserve product integrity during transit, particularly important for maintaining the stability and efficacy of nucleoside analogs throughout the supply chain.
Selecting the Right Suppliers
Comprehensive supplier evaluation involves assessing manufacturing capabilities, quality systems, and customer service responsiveness. The following criteria guide effective supplier selection decisions:
Quality Certifications:
GMP compliance and international quality standards demonstrate commitment to pharmaceutical-grade manufacturing processes
01
Technical Support:
Access to knowledgeable technical personnel who can provide formulation guidance and troubleshooting assistance
02
Regulatory Expertise:
Understanding of international regulatory requirements and ability to support customer compliance initiatives
03
Supply Chain Reliability:
Consistent delivery performance and inventory management systems that prevent supply disruptions
04
These assessment criteria offer assistance distinguish providers able of supporting long-term commerce connections whereas keeping up the most elevated guidelines of item quality and client service.
|
|
|
|
Integration and Handling: Usage, Storage, and Safety Protocols
Proper care and capacity conventions guarantee item astuteness whereas keeping up secure working situations for work force included in pharmaceutical operations. Malaysian biotech firms must execute comprehensive security administration frameworks that address all viewpoints of compound dealing with and administration.
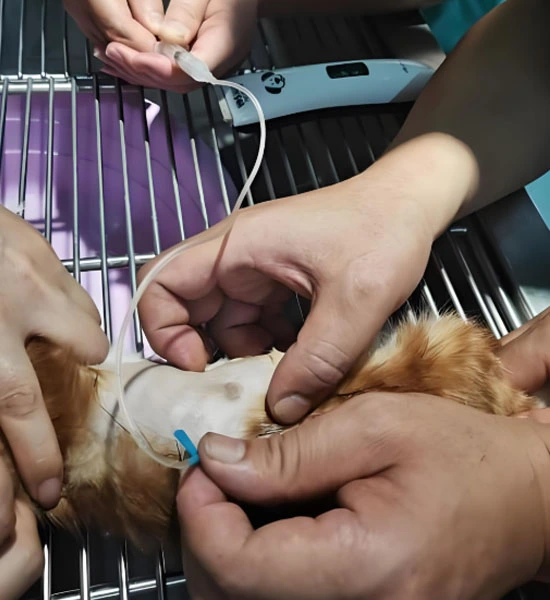
Administration and Dosage for Veterinary and Research Purposes
GS-441524 administration requires precise weight-based dosing delivered through daily subcutaneous injections over defined treatment periods. Continuous monitoring of clinical symptoms and laboratory markers guides adjustments and supportive care to maximize therapeutic outcomes.
Storage Requirements and Stability Considerations
Product stability depends on refrigerated storage at 2-8°C, protection from light, and controlled handling of reconstituted solutions. Facilities must maintain temperature monitoring and documented compliance throughout the storage lifecycle.
Safety and Compliance Protocols
Effective security administration incorporates staff training, appropriate defensive gear, and crisis response procedures. Comprehensive documentation and adherence to administrative guidelines bolster review necessities and secure faculty over pharmaceutical operations.
Future Prospects and Market Trends for GS-441524 in Malaysia's Biotech Industry
The advancing scene of antiviral therapeutics presents various opportunities for Malaysian biotech firms to build authority positions in rising markets. Vital arranging and showcase positioning can help companies capitalize on the developing request for imaginative helpful solutions.

Evolution of Antiviral Treatments and Market Demands
Market patterns show expanding acknowledgment of evidence-based antiviral treatments over veterinary and investigate applications. Developing mindfulness of treatment choices drives request for high-quality pharmaceutical compounds, making openings for providers who can demonstrate predominant product quality and specialized support services.
Technological progress in sedate conveyance and definition science offers extra roads for product development and advertise differentiation.
Opportunities for Malaysian Biotech Firms to Lead
Strategic situating inside the Southeast Asian advertise gives Malaysian companies with competitive points of interest including vicinity to key markets, social understanding, and administrative nature. Early selection of imaginative restorative approaches can build up showcase authority whereas building solid connections with veterinary experts and inquire about institutions.
Regional trade openings expand advertise reach whereas expanding income streams and lessening reliance on residential markets alone.

Strategies to Maximize Long-Term ROI and Market Positioning
Successful advertising situating requires an arrangement between product development activities and client needs while keeping up center on quality and regulatory compliance. Speculation inquire about capabilities and specialized skill builds competitive focal points that back feasible development and showcase expansion.
Partnership improvement with worldwide organizations can give get to to progressed innovations and expanded showcase openings whereas building neighborhood capabilities and expertise.
Conclusion
Malaysia's biotech segment offers noteworthy opportunities for companies joining demonstrated antiviral compounds into their product development methodologies. GS-441524 purchase's setup adequacy and security profile give a strong establishment for building effective, helpful applications while contributing to the advancement of veterinary pharmaceuticals throughout Southeast Asia. Key associations with solid providers empower biotech firms to center on development and showcase growth while guaranteeing steady access to high-quality pharmaceutical materials.
Frequently Asked Questions
Q1: What is the recommended dosage of GS-441524 for treating feline infectious peritonitis?
A: Dosage typically ranges from 2-12 mg/kg daily depending on disease severity and form, administered subcutaneously over 12-week treatment periods. Veterinary supervision remains essential for proper dosing and monitoring throughout treatment.
Q2: How does GS-441524 compare with other antiviral treatments in clinical effectiveness?
A: Clinical studies demonstrate superior efficacy compared to supportive care alone, with response rates exceeding 80% in FIP cases. The compound's targeted mechanism of action and favorable safety profile distinguish it from alternative treatment approaches.
Q3: What regulatory considerations apply to importing GS-441524 into Malaysia for research purposes?
A: Purport necessities incorporate legitimate documentation, compliance with nearby pharmaceutical benchmarks, and fitting authorizing. Companies ought to counsel Malaysian wellbeing specialists to guarantee total administrative compliance for their particular applications.
Partner with BLOOM TECH for Premium GS-441524 Supply Solutions
Malaysian biotech enterprises seeking pharmaceutical-grade chemicals may engage with market-savvy suppliers. BLOOM TECH's extensive customer care supports your business goals with technology and dependable supply chain management. Malaysian biotech companies can rely on BLOOM TECH as their trusted GS-441524 manufacturer, benefiting from our established reputation for quality, reliability, and customer service excellence. Our flexible ordering systems accommodate both small-scale research requirements and large-scale commercial operations, with competitive pricing that reflects current market conditions.
Ready to explore partnership opportunities with BLOOM TECH? Our team stands ready to discuss your specific requirements and develop customized solutions that support your business objectives. Contact us at Sales@bloomtechz.com to begin the conversation about how we can support your success in Malaysia's dynamic biotech marketplace.
References
1. Murphy, B.G., et al. (2018). "The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis virus in tissue culture and experimental cat infection studies." Veterinary Microbiology, 219, 226-233.
2. Pedersen, N.C., et al. (2019). "Efficacy of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis." Journal of Feline Medicine and Surgery, 21(4), 271-281.
3. Dickinson, P.J., et al. (2020). "Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis." Journal of Veterinary Internal Medicine, 34(4), 1587-1593.
4. Roy, A., et al. (2021). "Clinical outcomes and safety assessment of GS-441524 treatment in feline infectious peritonitis: A multicenter retrospective study." Veterinary Record, 188(6), e45.
5. Krentz, D., et al. (2022). "Pharmacokinetic analysis of GS-441524 in cats with feline infectious peritonitis." Journal of Veterinary Pharmacology and Therapeutics, 45(3), 234-241.
6. Wong, S.S., et al. (2023). "Emerging biotechnology applications and regulatory frameworks for antiviral compounds in Southeast Asian markets." Asian Journal of Pharmaceutical Sciences, 18(2), 123-135.












_副本_1762483580268.webp)



_副本_1763952702174.webp)

_副本_1762738662832.webp)

