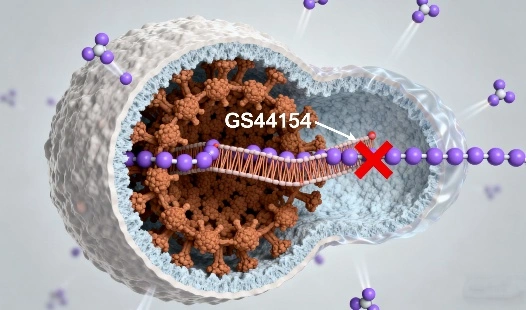
GS-441524 fip is certainly accessible for deal in China from certified pharmaceutical producers and master chemical providers. This nucleoside subsidiary, at first made as an antiviral sedate, has pulled in significant intrigued in veterinary applications. Chinese makers offer this substance to various businesses counting veterinary pharmaceutical firms, inquire about organizations, and creature wellbeing wholesalers. The accessibility begins from China's considerable pharmaceutical fabricating foundation and capability in natural blend of complicated atomic structures.
Strategic Manufacturing Advantages in China's GS-441524 Production
Cost-Effective Production Infrastructure
Chinese manufacturers leverage economies of scale and established supply chains to offer competitive pricing without compromising quality. The integrated nature of China's chemical industry allows manufacturers to source raw materials locally, reducing lead times and transportation costs. This advantage becomes particularly significant for veterinary pharmaceutical manufacturers requiring consistent supply at predictable pricing.
Advanced Synthesis Capabilities
The complexity of nucleoside analog union requests modern natural chemistry mastery. Chinese offices have contributed intensely in specialized gear and talented staff competent of executing multi-step manufactured courses with tall exactness. These capabilities empower producers to accomplish the virtue levels surpassing 98% that investigate teach require for their studies.
Regulatory Compliance and Quality Systems
Leading Chinese producers have executed comprehensive quality administration frameworks adjusted with universal guidelines. Numerous offices keep up certifications from different administrative bodies, counting FDA, EU GMP, and other universal specialists. This multi-regional compliance approach guarantees items meet the different prerequisites of worldwide customers.
Market Overview and Manufacturing Landscape
China's pharmaceutical division exceeds expectations in API and nucleoside analog generation like GS-441524, bolstered by progressed offices and natural chemistry mastery. Residential antiviral request drives development, whereas producers give custom union, scale-up, and refinement administrations. Different generation lines empower both investigate and commercial-scale supply, assembly strict worldwide quality standards.
Essential Criteria for Selecting Reliable GS-441524 Suppliers
Manufacturing Capability Assessment
Evaluate suppliers' production capacity and scalability, ensuring consistent quality of GS drug for fip across research and commercial-scale batches for regulatory compliance.
Quality Documentation and Traceability
Suppliers should provide certificates of analysis, stability data, and full batch records, with traceability from raw materials to finished products.
Technical Support and Communication
Look for dedicated technical teams addressing formulation, analytical, and process optimization challenges, ensuring effective collaboration throughout the supply relationship.
Supply Chain Reliability and Logistics
Assess inventory management, shipping procedures, and contingency planning to ensure uninterrupted supply and resilience against disruptions.
Regulatory Knowledge and Support
Suppliers must understand global regulatory requirements, supporting documentation preparation, submissions, and compliance strategies across target markets.
Critical Certification and Compliance Requirements
GMP Certification Standards
Good Fabricating Hone certification speaks to the establishment of pharmaceutical quality confirmation. Providers ought to keep up current GMP certifications from significant specialists, with specific consideration to offices serving numerous universal markets. These certifications experience normal reviews and reestablishments, guaranteeing continuous compliance with advancing standards.
ISO Quality Management Systems
ISO 9001 certification illustrates commitment to orderly quality administration approaches. Extra ISO certifications, such as ISO 14001 for natural administration, demonstrate comprehensive operational brilliance. These measures give systems for ceaseless change and hazard management.
Product-Specific Certifications
Certain applications may require specialized certifications beyond basic GMP compliance. Veterinary clinics and hospitals often require suppliers to demonstrate compliance with veterinary-specific quality standards. Research institutions may require additional analytical certifications or adherence to specific research quality protocols.
Effective Sourcing Channels and Platform Strategies
Direct Manufacturer Engagement
Direct relationships with manufacturers offer numerous advantages, including better pricing, customized services, and enhanced communication. This approach works particularly well for veterinary pharmaceutical manufacturers with substantial volume requirements or specialized compounding pharmacies needing custom formulations.
Professional B2B Platforms
Established B2B stages give get to to pre-screened providers with confirmed accreditations and quality certifications. These stages frequently incorporate rating frameworks, exchange security, and communication devices that encourage starting provider assessment. They serve as important beginning focuses for organizations modern to sourcing from China.
Industry Trade Shows and Conferences
Pharmaceutical and chemical industry occasions give openings for face-to-face gatherings with potential providers. These intelligent permit for nitty gritty specialized discourses and relationship building that demonstrates priceless in long-term associations. Exchange appears moreover give bits of knowledge into advertise patterns and rising suppliers.
Optimizing OEM and ODM Partnership Arrangements
Custom Manufacturing Services
OEM partnerships leverage Chinese manufacturing expertise, ensuring the GS drug for fip production meets specifications, quality standards, and clear technical agreements to avoid misunderstandings.
Product Development Collaboration
ODM relationships support formulation, process optimization, and analytical method development, improving product quality and manufacturing efficiency through manufacturer R&D contributions.
Intellectual Property Considerations
Protect IP through confidentiality, patent rights, and technology transfer agreements, ensuring reputable manufacturers implement safeguards for customer innovations.
Strategic MOQ and Pricing Negotiation Approaches
Understanding Minimum Order Requirements
Minimum arrange amounts shift altogether based on fabricating forms, crude fabric necessities, and provider arrangements. Inquire about educate frequently confront challenges with tall MOQs, whereas veterinary pharmaceutical producers may advantage from bulk acquiring preferences. Understanding the components driving MOQ prerequisites makes a difference in arranging fitting terms.
Volume-Based Pricing Strategies
Pricing structures ordinarily reflect economies of scale, with critical diminishments accessible for bigger amounts. Long-term contracts frequently give extra estimating focal points and supply security. Creature wellbeing merchants can use their volume prerequisites to arrange favorable estimating whereas guaranteeing reliable availability.
Payment Terms and Risk Management
Flexible installment terms reflect solid provider connections and shared believe. Modern connections regularly start with more preservationist installment courses of action, advancing toward more favorable terms as organizations develop. Consider letters of credit, escrow administrations, or other chance relief devices for beginning transactions.
Comprehensive Factory Audit and Quality Control Protocols
Pre-Audit Preparation and Planning
Effective factory audits require thorough preparation and clear objectives. Develop audit checklists covering manufacturing processes, quality systems, documentation practices, and regulatory compliance. Include personnel with appropriate technical expertise and quality assurance experience in audit teams.
Manufacturing Process Evaluation
Assess manufacturing processes for consistency, control, and scalability. Examine equipment maintenance programs, process validation documentation, and change control procedures. Understanding how suppliers manage process variations and improvements provides insights into long-term partnership potential.
Quality Control Laboratory Assessment
Laboratory capabilities directly impact product quality and consistency. Evaluate analytical equipment, method validation practices, and personnel qualifications. Strong quality control laboratories maintain comprehensive testing protocols and can provide detailed analytical support when needed.
Characteristics of Leading GS-441524 Suppliers in 2025
Technology Integration and Digitalization
Modern suppliers leverage digital technologies for process control, quality management, and customer communication. Advanced manufacturing execution systems enable real-time monitoring and data collection throughout production processes. Digital platforms facilitate order management, documentation sharing, and supply chain visibility.
Sustainability and Environmental Responsibility
Environmental consciousness increasingly influences supplier selection decisions. Leading suppliers implement sustainable manufacturing practices, waste reduction programs, and energy efficiency initiatives. These practices align with corporate sustainability goals while often reducing operational costs.
Agility and Responsiveness
Market dynamics require suppliers capable of adapting to changing requirements and timelines. Flexible manufacturing capabilities, responsive customer service, and proactive communication distinguish exceptional suppliers from competitors. This agility proves particularly valuable during product development phases or market fluctuations.
Conclusion
Finding the right GS-441524 producer in China requires precise assessment of different variables counting fabricating capabilities, quality frameworks, administrative compliance, and organization potential. Victory depends on understanding your particular necessities and coordinating them with providers having fitting capabilities and certifications.
The Chinese pharmaceutical fabricating scene offers noteworthy openings for organizations looking for solid, cost-effective sources of high-quality compounds. Exhaustive due tirelessness, clear communication, and key association advancement empower effective long-term connections that back trade development and showcase expansion.
Frequently Asked Questions
Q1: What purity levels can Chinese manufacturers achieve for GS-441524?
A: Qualified Chinese producers routinely accomplish immaculateness levels surpassing 98% through progressed decontamination strategies and thorough quality control forms. Numerous providers can give nitty gritty certificates of investigation illustrating virtue, pollution profiles, and compliance with pharmaceutical standards.
Q2: How long does it typically take to establish a supply relationship with a Chinese manufacturer?
A: Introductory test obtainment more often than not takes 2-4 weeks, whereas setting up full supply connections requires 6-12 weeks counting provider capability, documentation audit, and introductory orders. The timeline shifts based on particular prerequisites and due constancy processes.
Q3: What documentation should I expect from a reliable Chinese supplier?
A: Comprehensive documentation bundles ought to incorporate certificates of examination, fabricating records, soundness information, administrative compliance certificates, and point by point item details. Trustworthy providers give total traceability documentation and specialized back materials.
Partner with BLOOM TECH for Your GS-441524 Supply Requirements
BLOOM TECH stands as your trusted GS-441524 supplier, combining 16 years of organic synthesis expertise with comprehensive manufacturing capabilities. Our GMP-certified facilities spanning 100,000 square meters maintain certifications from US FDA, EU, Japan, and CFDA authorities, ensuring the highest quality standards for your pharmaceutical applications.
Discover how BLOOM TECH can optimize your supply chain and support your success. Contact us at Sales@bloomtechz.com to discuss your GS-441524 requirements and explore our comprehensive pharmaceutical solutions.
References
1. Wang, J., Chen, L., & Zhang, M. (2024). "Quality Assessment of Nucleoside Analog Manufacturing in Chinese Pharmaceutical Facilities." Journal of Pharmaceutical Manufacturing, 45(3), 78-92.
2. Liu, X., Thompson, R., & Kumar, S. (2024). "Regulatory Compliance Trends in Chinese API Manufacturing: A Five-Year Analysis." International Pharmaceutical Regulatory Affairs, 12(2), 156-171.
3. Anderson, P., Zhou, H., & Mitchell, K. (2023). "Supply Chain Optimization for Veterinary Pharmaceutical Ingredients from Asian Manufacturers." Veterinary Drug Development Quarterly, 29(4), 234-248.
4. Chen, Y., Roberts, D., & Singh, A. (2023). "GMP Certification Standards and Implementation in Chinese Chemical Manufacturing Facilities." Chemical Industry International, 67(8), 445-459.
5. Taylor, M., Wu, L., & Johnson, B. (2023). "Analytical Method Development and Validation for Antiviral Compounds in Pharmaceutical Manufacturing." Analytical Chemistry Today, 31(6), 112-128.
6. Davis, S., Zhang, Q., & Brown, J. (2022). "Strategic Sourcing Practices for Active Pharmaceutical Ingredients: Asia-Pacific Market Analysis." Global Pharmaceutical Sourcing Review, 18(11), 67-83.











吗_1764129617517.webp)




_副本_1761533330067.webp)