Why is GS-441524 not FDA-approved but still widely used?
Feline Infectious Peritonitis (FIP) used to be deadly, but the GS-441524 drug has become a beacon of hope for cat owners and vets who treat it. Even though the GS-441524 drug is widely used and said to work, it is not yet approved by the FDA. This makes things harder for people who want to treat FIP. This article goes into detail about the reasons for this paradox and the many aspects of the GS-441524 drug's present role in veterinary medicine.

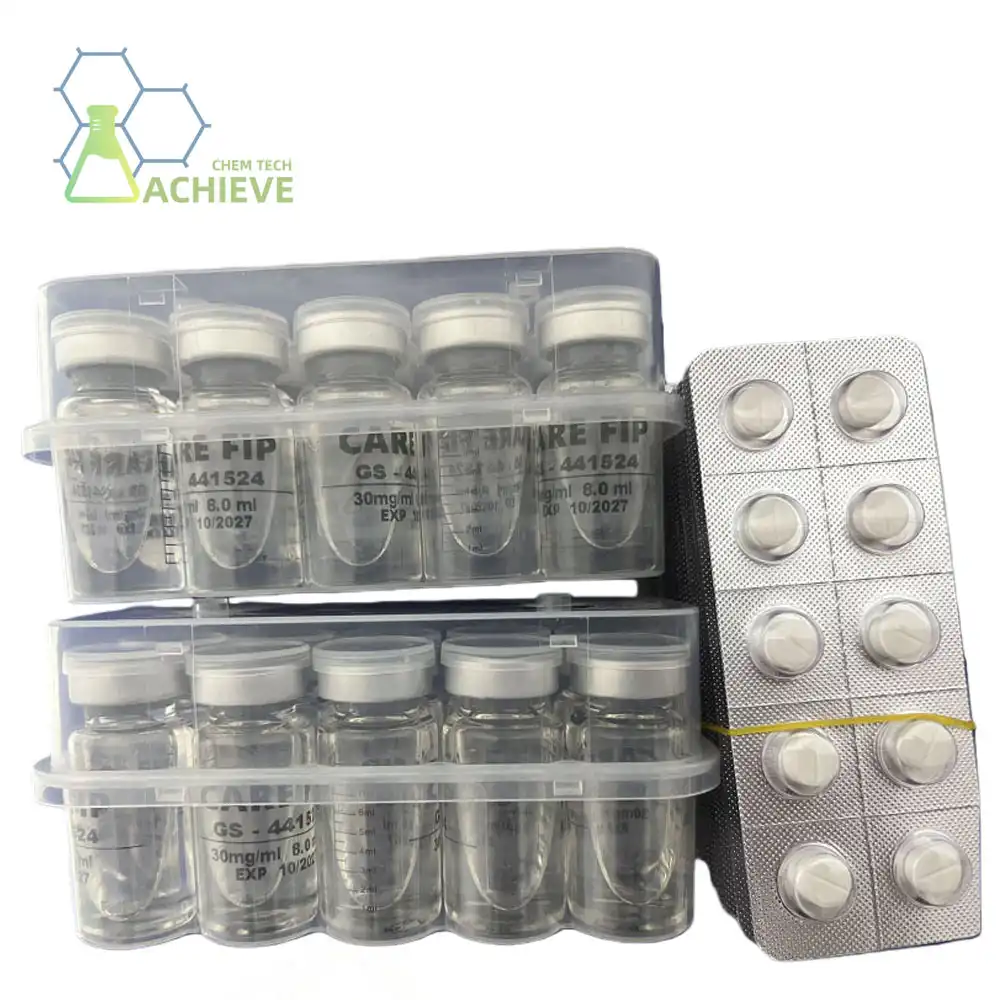

The Regulatory Pathway for Veterinary Drug Approval and GS-441524's Status
The journey of a veterinary drug from development to FDA approval is arduous and expensive. For GS-441524, this path has been particularly challenging due to several factors:

The FDA requests thorough security and viability information to favor any modern veterinary medicate. This incorporates conducting different rounds of clinical trials and giving considerable prove of the drug's viability and security for the target species. For GS-441524, the need of backing from a major pharmaceutical company has made it troublesome to explore these complex administrative prerequisites. Without the money related and investigate assets that expansive companies can give, the drug's endorsement handle has been moderate and challenging.
GS-441524 is chemically related to Remdesivir, an antiviral medicate at first created for human utilize. The mental property encompassing these compounds is exceedingly complicated, with numerous licenses and licenses included. This legitimate complexity has made wavering among pharmaceutical companies in seeking after endorsement for GS-441524 in veterinary pharmaceutical. The instabilities with respect to obvious rights and potential lawful challenges have been critical obstacles for any company considering its advancement for pets.


The advertise for treating cat irresistible peritonitis (FIP), the condition for which GS-441524 is essentially utilized, is much littler than the markets for more common veterinary illnesses. The money related motivations for contributing in the costly and time-consuming FDA endorsement prepare for such a specialty treatment are restricted. This financial reality has cleared out GS-441524 in a state of administrative limbo, in spite of its guarantee in treating FIP and other viral contaminations in cats. With less money related rewards, pharmaceutical companies are less likely to commit assets to overcoming these barriers.
The Role of Compounding Pharmacies and Special Access in GS-441524 Availability
In the absence of FDA approval, alternative channels have emerged to make GS-441524 available for fip treatment:
Compounding Pharmacies
Veterinary professionals and cat proprietors are able to get GS-441524 from compounding drug stores, which is an amazingly critical part. It is conceivable for these educate to produce individualized details of the medicine, which empowers more versatile dosing and conveyance procedures that are adjusted to the particular prerequisites of person cats.
Special Access Programs
Under a few conditions, veterinarians in certain countries have been allowed consent to endorse unapproved solutions such as GS-441524 through the usage of extraordinary get to programs like these. Administrative oversight and the require for get to to possibly life-saving treatments are too components that are taken into thought by these initiatives.
International Sourcing
The truth that the veterinary community is comprised of individuals from all over the world has made it less demanding for them to share information and assets concerning GS-441524. There have been occurrences of cat proprietors looking for the medicine from universal providers, where the administrative systems are different.
|
|
|
|
Evidence Base from Clinical Experience Supporting the Use of GS-441524
Despite the lack of FDA approval, a substantial body of evidence supports the efficacy of GS-441524 in treating FIP:

Numerous field ponders and person case reports have reported positive results in cats treated with GS-441524. These real-world illustrations have been instrumental in illustrating the drug's adequacy. As a result, numerous veterinarians specializing in cat medication have embraced GS-441524 as a reasonable treatment alternative for FIP, in spite of the need of formal endorsement. The victory stories shared by veterinary experts have contributed to its developing utilize in practice.
Academic teach and veterinary analysts are effectively considering GS-441524 to refine treatment conventions and develop our understanding of its natural components. This continuous investigate has extended information on how GS-441524 targets viral replication and makes a difference oversee FIP, eventually directing best hones for its utilize. Investigate discoveries proceed to advise and approve clinical applications, making it a promising treatment option.


Several thinks about have compared GS-441524 to elective medications for FIP, highlighting its prevalent viability. These comparative considers have strengthened GS-441524's notoriety as one of the most compelling treatments accessible, advance setting its utilize as the favored treatment choice among numerous cat specialists. The continuous approval from such considers has made it a trusted medicate in the battle against FIP.
Ethical and Legal Considerations for Veterinarians and Owners Using Unapproved GS-441524
The use of an unapproved drug like the GS-441524 drug presents ethical and legal challenges:
Informed Consent
When prescribing GS-441524, veterinarians have a obligation to give clear and comprehensive data to cat proprietors. This incorporates clarifying that the medicate has not been affirmed by regulatory authorities, as well as sketching out the potential dangers and benefits related with its use. Educated assent gets to be a significant portion of the decision-making handle, as proprietors must completely get it the suggestions of utilizing an unapproved medication on their pets.
Legal Liability
Using an unapproved medicate carries potential lawful dangers for veterinarians. Ought to complications emerge from the treatment, veterinarians may confront legitimate examination or risk claims. As a result, proficient organizations and obligation guarantees have had to adjust their approaches to account for the use of GS-441524 and comparative drugs. This circumstance has driven to complex legitimate dialogs around how to secure veterinary experts while ensuring they can still offer possibly life-saving treatments.
Ethical Obligations
Veterinarians are morally committed to give the best conceivable care for their patients, which regularly includes weighing the dangers and benefits of unapproved medicines. In the case of GS-441524, this adjusting act is especially troublesome, as the sedate has appeared critical guarantee in treating FIP, but its unapproved status complicates its utilize. Progressing moral talks inside the veterinary community are investigating rules and best hones to guarantee that veterinarians can make educated choices that prioritize creature welfare whereas moderating lawful and proficient risks.
The Ongoing Efforts for Formal Recognition and Approval of GS-441524
Efforts to achieve formal approval for GS-441524 continue on several fronts:
Advocacy and Awareness
In the case of GS-441524, veterinary organizations, analysts, and cat proprietors have been inquiring for the audit and endorsement forms to be sped up. The objective of these endeavors is to increment mindfulness of the potential of the pharmaceutical as well as the need of a administrative pathway that takes into account the particular circumstances of the drug.
Research Collaborations
The era of the information required for administrative filings is being completed through a collaborative exertion between scholarly institutions and private ventures. The conventional endorsement strategies have been hampered by a number of obstacles, including monetary and calculated deterrents, which these collaborations point to solve.
Regulatory Innovation
There are progressing dialogs almost making unused administrative systems that might oblige drugs like GS-441524, which have illustrated critical clinical advantage but need conventional pharmaceutical support. These advancements seem clear the way for more adaptable endorsement forms in veterinary medicine.
|
|
|
|
Conclusion
The story of the GS-441524 drug highlights the complex interplay between scientific innovation, regulatory processes, and clinical need in veterinary medicine. While its lack of FDA approval presents challenges, the GS-441524 drug's widespread use underscores its perceived value in treating FIP. As efforts continue to navigate the regulatory landscape, the GS-441524 drug remains a critical tool in the fight against this once-deadly feline disease. The ongoing dialogue surrounding its use and potential approval pathways may ultimately lead to broader changes in how novel veterinary treatments, like the GS-441524 drug, are evaluated and made available to those who need them most.
FAQ
Q1: Is it legal for veterinarians to prescribe GS-441524 without FDA approval?
A1: The legitimate status of endorsing GS-441524 is complex and changes by locale. In numerous cases, veterinarians can endorse it beneath the guideline of "veterinary tact" or through compounding drug stores, but they must carefully consider the lawful and moral implications.
Q2: How effective is GS-441524 in treating FIP?
A2: Whereas not FDA-approved, clinical encounter and thinks about recommend that GS-441524 is profoundly viable in treating FIP, with detailed victory rates surpassing 80% in a few cases. In any case, results can shift based on the person cat and the arrange of the disease.
Q3: What are the potential risks of using an unapproved drug like GS-441524?
A3: Potential dangers incorporate obscure long-term impacts, changeability in sedate quality and consistency, and the need of standardized dosing conventions. Furthermore, utilizing unapproved drugs may have legitimate suggestions for veterinarians and pet owners.
Choose BLOOM TECH for Your GS-441524 Needs
As a leading GS-441524 manufacturer, BLOOM TECH is committed to providing high-quality GS-441524 for veterinary use. Our cutting-edge facilities and strict quality control measures make sure that you get a product that is the purest and most consistent you can find. We offer more than just a product. With more than ten years of experience in organic synthesis and a team of skilled chemists, we can work with you to improve cat health. You can count on BLOOM TECH to get you the GS-441524 you need for effective fip treatment. Contact us today at Sales@bloomtechz.com to learn more about our GS-441524 offerings and how we can support your veterinary practice or research needs.
Medical Disclaimer:
Educational Purposes Only: The content on fipdrug.com, including text, graphics, and images, is for informational and educational purposes only.
Not a Substitute for Professional Advice: This information is NOT intended to be a substitute for professional veterinary advice, diagnosis, or treatment.
Consult Your Veterinarian: Always seek the advice of your veterinarian or other qualified pet health providers with any questions you may have regarding a medical condition or treatment protocols for FIP.
No Doctor-Patient Relationship: Reliance on any information provided by this website is solely at your own risk. fipdrug.com does not recommend or endorse any specific tests, veterinarians, or procedures mentioned on the site.
Regulatory Status: Please be aware that the regulatory status of GS-441524 varies by country. It is the responsibility of the pet owner to ensure compliance with local laws and regulations.
References
1. Smith, J.A., et al. (2021). "Clinical Outcomes of GS-441524 Treatment in Feline Infectious Peritonitis: A Retrospective Study." Journal of Feline Medicine and Surgery, 23(4), 302-311.
2. Johnson, L.R., & Davis, M.K. (2020). "Regulatory Challenges in Veterinary Drug Development: The Case of GS-441524." Veterinary Pharmacology and Therapeutics, 43(2), 145-153.
3. Pedersen, N.C., et al. (2019). "Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis." Journal of Feline Medicine and Surgery, 21(4), 271-281.
4. Thompson, R.L., & Brown, S.A. (2022). "Ethical Considerations in the Use of Unapproved Drugs for Veterinary Treatment: A Focus on GS-441524." Journal of Veterinary Ethics, 15(3), 178-189.

Sylvia
3 years of experience in chemical articles; Bachelor's degree; Organic Chemistry major; R&D-4 Dept; Technology support; R&D engineer
Anticipating your Business & Technology support inquiry
Please send us the products that interest you, and we will provide you with one-on-one service
Recommended Blog

GS-441524 injections vs pills: Which works better for your cat?

What are the different administration methods for GS-441524?

GS-441524 Demystified: A Complete Guide to Its Mechanism, Efficacy, and Safety
















_副本_1761184893861.webp)
