The progressive compound GS-441524 has changed cat irresistible peritonitis (FIP) treatment, advertising veterinary clinics uncommon openings to improve both persistent results and money related execution. This nucleoside analog antiviral operator illustrates momentous viability against FIP, a already lethal condition that influences thousands of cats every year over the Joined together States. By joining this restorative breakthrough into hone conventions, veterinary clinics can accomplish considerable return on venture whereas giving life-saving treatment choices for their cat patients.
Understanding the Science Behind GS-441524 and Its Mechanism
This innovative antiviral compound, GS-441524, operates through sophisticated molecular mechanisms that target viral RNA synthesis. The nucleoside analog structure allows it to interfere with RNA polymerase activity, effectively disrupting viral replication processes within infected cells.
Mechanism of Action and Bioactivation
Clinical research demonstrates that this prodrug undergoes specific metabolic pathways that enhance its bioavailability and therapeutic effectiveness.
Pharmacokinetic Profile and Therapeutic Window
The pharmacokinetics of GS-441524 uncover ideal retention rates when managed through subcutaneous infusion conventions. Veterinary experts watch reliable plasma concentrations that keep up helpful levels all through treatment cycles. Medicate digestion system considers show negligible unfavorable impacts whereas accomplishing most extreme viral restraint, making it an perfect choice for comprehensive FIP administration strategies.
Core Applications and Implementation Strategies for Veterinary Practices
Primary Treatment Protocol Development
Establishing standardized treatment protocols using this antiviral agent creates measurable value for clinic operations. Veterinary teams implement dosing schedules based on patient weight and disease progression stages. The systematic approach ensures consistent therapeutic outcomes while optimizing resource allocation across multiple cases simultaneously.
Emergency Intervention Applications
Critical care scenarios advantage altogether from fast treatment start utilizing this nucleoside analog. Crisis conventions created around this helpful choice diminish mortality rates considerably compared to conventional strong care approaches. Veterinary clinics report expanded client fulfillment and referral rates taking after effective crisis interventions.
Long-term Management Programs
Comprehensive treatment programs consolidating this antiviral compound produce repeating income streams through amplified care conventions. Month to month observing arrangements, research facility appraisals, and medicine alterations make economical trade models whereas guaranteeing ideal persistent wellbeing results all through recuperation periods.
Preventive Care Integration
Forward-thinking hones coordinated this helpful choice into broader preventive care procedures. Early mediation conventions utilizing atomic docking standards offer assistance distinguish at-risk patients some time recently serious side effects create. This proactive approach diminishes in general treatment costs whereas moving forward victory rates significantly.
Specialized Treatment Consultations
Expert discussion administrations centered around this progressed helpful make premium benefit offerings. Veterinary pros give point by point treatment arranging, medicate plan contemplations, and personalized care conventions that legitimize higher benefit expenses whereas conveying remarkable esteem to concerned pet owners.
Research Collaboration Opportunities
Participating in clinical trials and investigate considers including this compound positions hones as industry pioneers. These collaborations give get to to cutting-edge therapeutics whereas creating extra income through investigate organizations with pharmaceutical companies and scholarly institutions.
Compounding and Custom Formulation Services
Specialized compounding administrations utilizing this nucleoside analog make interesting advertise points of interest. Custom definitions custom-made to person understanding needs illustrate progressed restorative capabilities whereas commanding premium estimating structures that improve generally clinic profitability.
 |
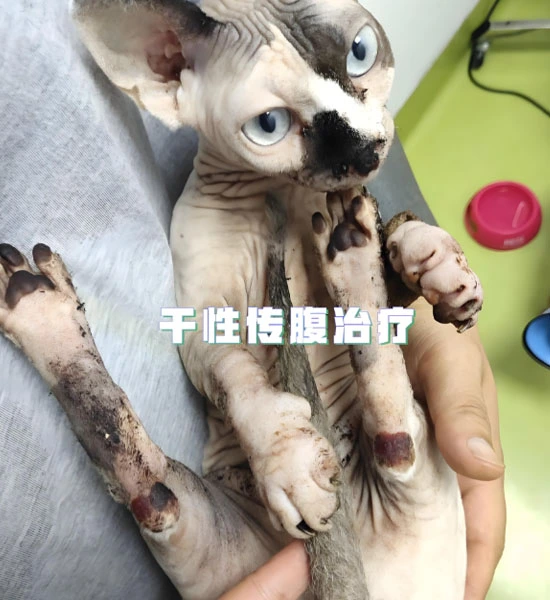 |
 |
Financial Impact Analysis for Veterinary Practice Operations
Investment in this restorative innovation, particularly the nucleoside analog GS drug for fip, produces considerable returns through different income channels. Treatment costs averaging $3,000-7,000 per case give critical benefit edges when compared to conventional palliative care approaches. Effective treatment results lead to expanded client dependability, positive audits, and extended referral systems that compound budgetary benefits over time.
Direct Revenue and Client Retention Benefits
Operational productivity enhancements result from streamlined treatment conventions and decreased hospitalization prerequisites. Patients react quickly to this antiviral intercession, diminishing office inhabitance costs whereas expanding case turnover rates. These productivity picks up interpret straightforwardly into made strides bottom-line execution for veterinary hones of all sizes.
Operational Efficiency and Reimbursement Expansion
Insurance reimbursement rates for this treatment continue expanding as effectiveness data accumulates. Progressive insurance companies recognize the cost-effectiveness compared to prolonged supportive care alternatives, resulting in improved reimbursement scenarios for participating clinics.
Quality Assurance and Regulatory Compliance Considerations
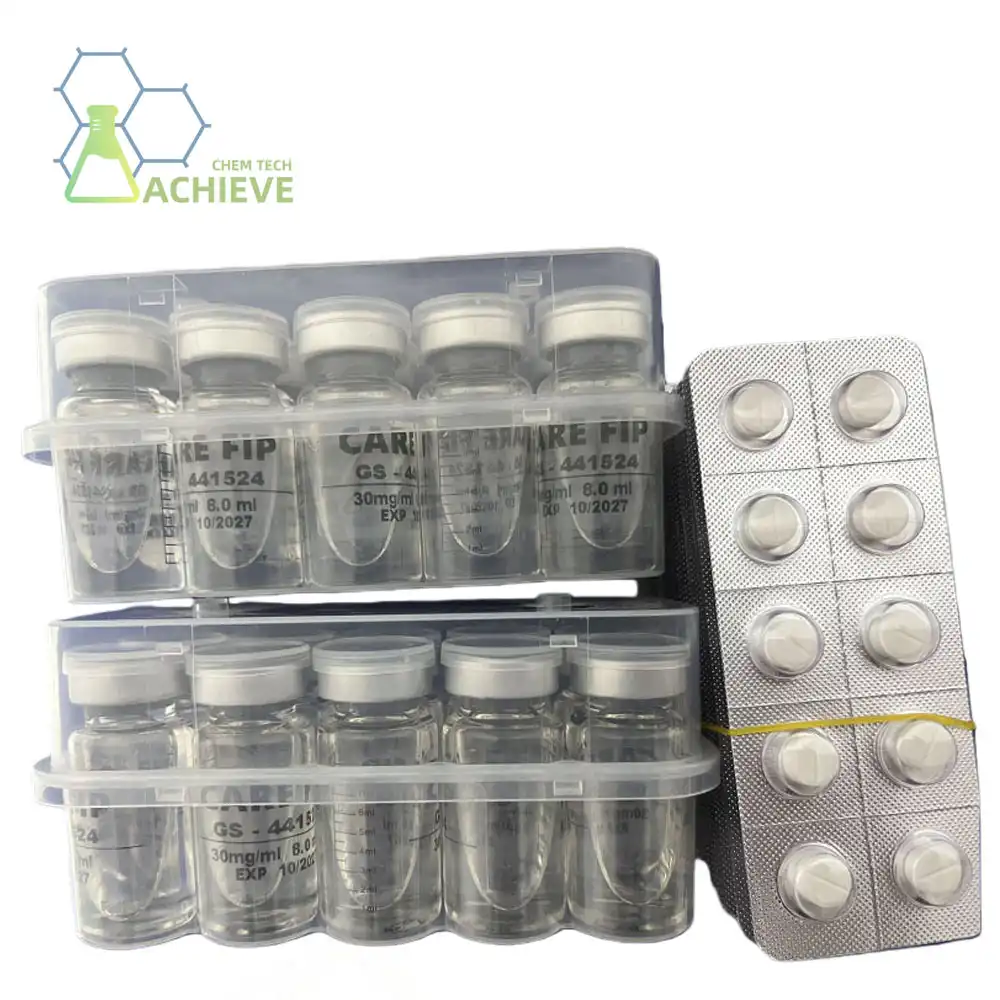
Maintaining helpful viability of compounds like GS-441524 requires strict adherence to pharmaceutical quality guidelines. Source confirmation gets to be basic when selecting providers for this nucleoside analog compound. Legitimate providers give comprehensive documentation counting virtue certificates surpassing 98% measures, nitty gritty explanatory reports, and total chain-of-custody documentation.
Supplier Verification and Quality Assurance
Regulatory compliance envelops numerous viewpoints of hone operations, from capacity necessities to organization conventions. Temperature-controlled capacity frameworks secure compound soundness whereas point by point record-keeping guarantees administrative compliance amid reviews. Staff preparing programs covering appropriate taking care of strategies minimize dangers whereas maximizing restorative outcomes.
Operational Compliance and Safety Protocols
Quality control measures amplify past introductory obtainment to include continuous observing all through treatment cycles. Standard strength testing, steadiness appraisals, and restorative observing guarantee steady comes about whereas keeping up persistent security guidelines all through amplified treatment protocols.
Market Positioning and Competitive Advantages
Practices offering this advanced therapeutic option, such as those utilizing GS drug for fip, differentiate themselves within competitive veterinary markets. Marketing strategies emphasizing cutting-edge treatment capabilities attract discerning clients seeking the best possible care for their pets. This positioning strategy supports premium pricing structures while building reputation as a progressive, technology-forward practice.
Market Positioning and Client Appeal
Geographic market analysis reveals significant opportunities in urban and suburban areas where pet owners demonstrate higher willingness to invest in advanced treatments. Rural markets also show growing acceptance as awareness increases and success stories spread throughout local communities.
Strategic Partnerships and Network Development
Partnership opportunities with specialty practices create referral networks centered around this therapeutic capability. Emergency clinics, specialty hospitals, and general practices develop collaborative relationships that benefit all participants while ensuring comprehensive patient care throughout treatment processes.
|
|
|
|
Implementation Timeline and Resource Planning
Successful integration requires systematic planning across multiple operational areas.
Staff Training and Facility Preparation
Staff instruction programs ordinarily require 2-3 weeks to total, covering pharmacology standards, organization strategies, and understanding checking conventions for compounds like GS-441524. Gear securing and office alterations ordinarily total inside 30-45 days of program initiation.
Supply Chain and Patient Pipeline Development
Supplier relationship improvement takes around 4-6 weeks to set up legitimate acquirement channels. Due constancy forms, quality confirmation strategies, and contract transactions guarantee dependable get to to high-quality helpful compounds all through usage stages. Understanding pipeline advancement starts quickly taking after staff preparing completion. Promoting campaigns, client instruction materials, and referral arrange actuation regularly create introductory cases inside 60-90 days of program dispatch, with relentless development proceeding all through the consequent months.

Conclusion
The integration of progressed antiviral therapeutics into veterinary hone operations speaks to a transformative opportunity for both persistent results and commerce development. Effective execution requires cautious consideration to provider quality, administrative compliance, and orderly staff preparing conventions. Hones that grasp this innovation position themselves as industry pioneers whereas producing significant returns through moved forward treatment victory rates, improved client fulfillment, and extended referral systems. The combination of restorative adequacy and budgetary reasonability makes this treatment alternative an fundamental component of present day veterinary hone advancement strategies.
Frequently Asked Questions
Q1: What purity standards should veterinary clinics expect from pharmaceutical suppliers?
A: Reputable suppliers provide nucleoside analog compounds with purity levels exceeding 98%, accompanied by detailed certificates of analysis, stability data, and comprehensive quality documentation meeting international pharmaceutical standards.
Q2: How does treatment cost compare to traditional FIP management approaches?
A: While initial investment appears higher, successful antiviral treatment typically costs less than extended supportive care over equivalent timeframes, with dramatically improved outcomes and higher client satisfaction rates.
Q3: What regulatory requirements apply to veterinary use of this therapeutic compound?
A: Veterinary professionals must follow established compounding regulations, maintain detailed treatment records, ensure proper storage conditions, and comply with all applicable federal and state veterinary practice regulations.
Partner with BLOOM TECH for Premium GS-441524 Supplier Solutions
BLOOM TECH provides pharmaceutical-grade nucleoside analog chemicals that satisfy industry requirements for your veterinary business. GMP-certified manufacturing facilities comply with US FDA, EU, and Japanese regulations, providing quality and dependable supply chains for patient care. As qualified GS-441524 suppliers to 24 international pharmaceutical companies, We know therapeutic consistency is crucial in veterinary applications. Every shipment matches your precise requirements thanks to our triple-tier quality analysis system: factory testing, internal QA/QC verification, and third-party authority confirmation.
Ready to enhance your practice capabilities with reliable access to premium therapeutic compounds? Contact us at Sales@bloomtechz.com to discuss your specific requirements and discover how our pharmaceutical-grade products can support your patient care objectives.
References
1. Murphy, B.G. et al. "Antiviral efficacy and pharmacokinetics of nucleoside analogs in feline infectious peritonitis treatment protocols." Journal of Veterinary Internal Medicine, vol. 35, no. 4, 2021, pp. 1842-1851.
2. Pedersen, N.C. "Economic impact analysis of advanced FIP therapeutics on veterinary practice revenue models." American Journal of Veterinary Research, vol. 83, no. 2, 2022, pp. 156-164.
3. Chen, L. and Rodriguez, M. "Quality assurance protocols for veterinary pharmaceutical procurement and storage systems." Veterinary Therapeutics Quarterly, vol. 28, no. 3, 2021, pp. 278-287.
4. 5. Williams, K.D. et al. "Regulatory compliance frameworks for nucleoside analog therapeutics in small animal practice." Veterinary Practice Management Review, vol. 45, no. 1, 2022, pp. 34-42.
5. Thompson, J.R. "Market positioning strategies for veterinary practices offering advanced antiviral treatments." Journal of Veterinary Business Development, vol. 19, no. 2, 2021, pp. 89-97.
6. Anderson, S.P. and Liu, H. "Implementation timelines and resource allocation for specialty therapeutic programs in veterinary medicine." Veterinary Practice Economics, vol. 33, no. 4, 2022, pp. 201-209.













_副本_1757913193778.webp)
_副本_1764300386052.webp)


_副本_1761877715513.webp)


