Proper storage of GS-441524 injection and tablet formulations is fundamental to maintaining their therapeutic efficacy and safety profile in veterinary applications. These antiviral compounds require specific environmental conditions to preserve their molecular integrity and pharmacological activity. Storage protocols for GS-441524 injection typically involve refrigeration between 2-8°C, while tablet formulations maintain stability at controlled room temperatures of 15-25°C with protection from light exposure and moisture. Understanding these critical storage parameters ensures optimal drug performance throughout the supply chain, from manufacturing facilities to veterinary clinics and research institutions.


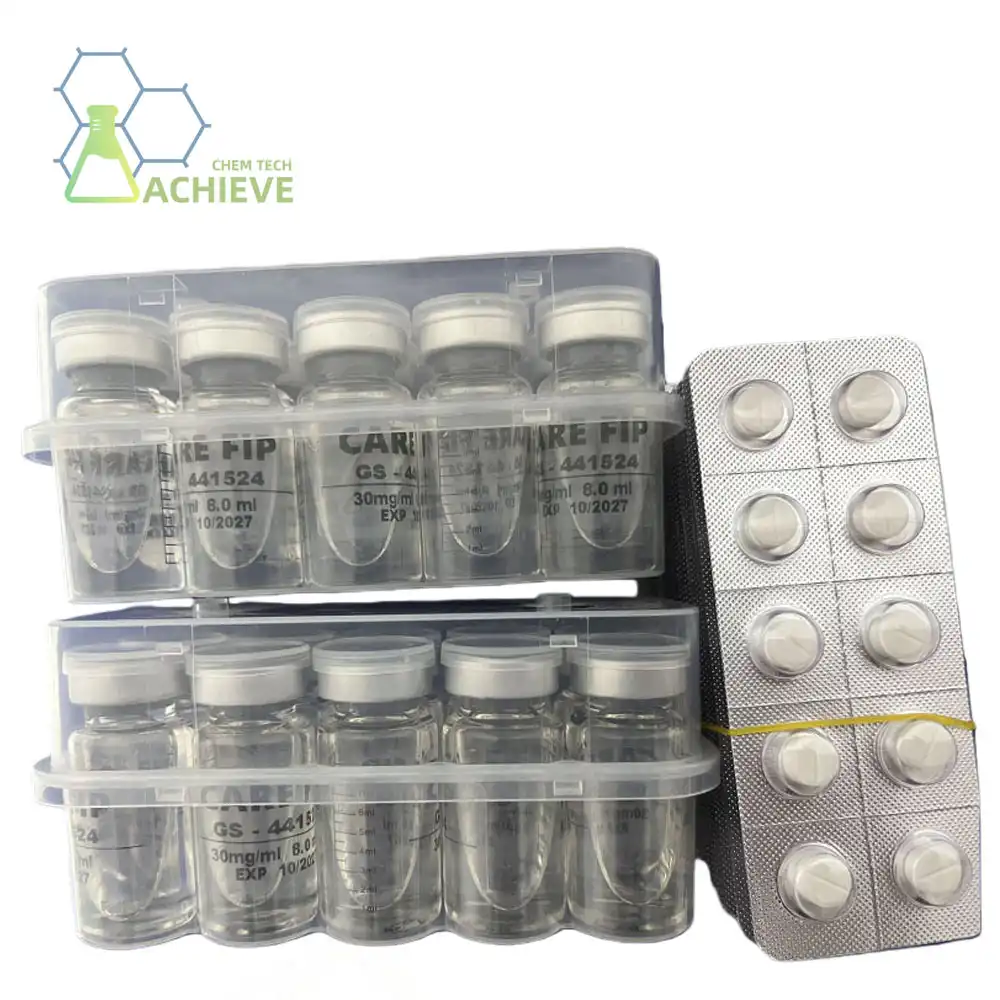
Understanding the Importance of Proper Storage for GS-441524

Compound Stability and Importance
GS-441524 speaks to a noteworthy progression in veterinary antiviral treatment, especially for cat wellbeing applications. The compound's chemical structure makes it helpless to debasement when uncovered to inappropriate natural conditions, which can drastically decrease its restorative adequacy. Guaranteeing the solidness of this particle from generation to organization is hence fundamental, as compromised astuteness specifically impacts clinical results and underscores the significance of educated dealing with all through the supply chain.
Consequences of Improper Storage
When GS-441524 formulations are stored incorrectly, several detrimental effects occur. Temperature fluctuations can accelerate molecular breakdown, leading to reduced active ingredient concentration and compromised antiviral activity. Light exposure, particularly ultraviolet radiation, can trigger photochemical reactions that alter the compound's molecular structure. Moisture infiltration can cause hydrolysis reactions, while improper packaging allows oxygen exposure that may oxidize sensitive functional groups. A careful GS-441524 purchase must consider the seller's storage and shipping protocols to avoid receiving a degraded product.

Key Degradation Factors
The steadiness profile of GS-441524 depends on different natural variables working synergistically. Temperature control remains the essential thought, as enzymatic and chemical corruption rates ordinarily twofold for each 10°C temperature increment. Mugginess levels over 60% relative mugginess can start moisture-related corruption pathways, whereas introduction to fluorescent or coordinate daylight can diminish power by up to 15% inside weeks of inappropriate capacity. This transaction requires strict natural control at each arrange to protect the compound's restorative value.
Recommended Storage Conditions for GS-441524 Injections and Tablets
Injectable definitions of GS-441524 request exacting cold chain administration to protect their pharmaceutical judgment. These fluid arrangements require steady refrigeration temperatures between 2-8°C, with temperature observing frameworks to guarantee compliance. The limit temperature run anticipates crystallization at lower temperatures whereas maintaining a strategic distance from quickened corruption at higher ranges.
Storage protocols for injectable formulations include several critical components:
Dedicated pharmaceutical refrigeration units with backup power systems and temperature alarms to maintain consistent cooling
01
Temperature mapping validation to identify cold spots and ensure uniform cooling throughout storage areas
02
Insulated packaging during transportation with temperature data loggers to maintain cold chain integrity
03
Segregated storage areas to prevent contamination from other pharmaceutical products or laboratory materials
04
These comprehensive capacity measures guarantee that injectable GS-441524 maintains its restorative power throughout dissemination channels, supporting dependable treatment results in clinical applications.
Tablet definitions offer more prominent capacity adaptability while still requiring controlled natural conditions. Room temperature capacity between 15-25°C gives ideal soundness for strong measurement shapes. The crystalline structure of tablet definitions offers inborn security against natural stressors, in despite the fact that appropriate bundling remains essential.
Light assurance speaks to another significant capacity thought for both definitions. Golden glass holders or dark bundling materials viably piece hurtful wavelengths that can corrupt dynamic fixings. Capacity zones ought to minimize presentation to fluorescent lighting and totally dispense with coordinate daylight access.
Moisture control through desiccant parcels or humidity-controlled situations avoids hydrolytic corruption. Relative mugginess levels ought to stay underneath 60% to anticipate dampness retention and keep up item solidness all through the rack life period.
Best Practices in Handling and Administration Preparation
Post-reconstitution handling of GS-441524 injection requires immediate attention to storage protocols. Once reconstituted from powder form or diluted for administration, the solution becomes more vulnerable to degradation and contamination. Reconstituted solutions typically maintain stability for 24-48 hours under refrigerated conditions, though specific formulations may have shorter or longer stability windows.
Professional dealing with conventions emphasizes the aseptic method during reconstitution and arrangement. Clean room situations or laminar stream hoods give suitable conditions for arrangement planning. Sterile diluents and appropriate blending methods guarantee homogeneous dispersion of dynamic fixings without presenting contaminants that seem to compromise quiet safety.
Bulk acquisition and conveyance require advanced coordination to keep up item keenness. Cold chain shipping utilizes approved bundling frameworks with gel packs or dry ice to keep up temperature ranges amid travel. Temperature checking gadgets track natural conditions all through shipping, giving documentation for quality affirmation purposes.
International shipping controls for pharmaceutical items require comprehensive documentation counting certificates of investigation, soundness information, and administrative compliance data. Bundling must meet worldwide transportation guidelines whereas keeping up item steadiness over changing climate conditions and shipping durations.
|
|
|
|
Common Storage Mistakes and How to Avoid Them

Critical Storage Conditions
Misinterpretation of storage requirements leads to preventable product degradation and therapeutic failures, a risk particularly evident with temperature-sensitive items like a GS-441524 purchase. Room temperature storage confusion represents a frequent error, as ambient conditions in warmer climates or poorly controlled facilities often exceed the strict 20-25°C range required for true "controlled room temperature." This passive reliance on ambient conditions, without active environmental management, directly compromises molecular stability and potency, rendering treatments ineffective before their stated expiration date.
Packaging and Handling Integrity
Product stability is equally dependent on maintaining original packaging integrity, even when temperature is controlled. Violations such as opening sealed containers prematurely or transferring contents expose the product to destabilizing environmental factors like moisture and oxygen, which accelerate chemical degradation. For any pharmaceutical, including a GS-441524, using non-original, unapproved containers eliminates crucial protective barriers, compromises sterility, and removes essential labeling, thereby creating significant safety risks and potential dosing errors alongside the loss of efficacy.


Systematic Management Protocols
Preventing storage-related failures requires robust systematic protocols. Inventory management must enforce strict first-in-first-out rotation to prevent expiration, while regular monitoring using calibrated data loggers verifies continuous compliance with environmental parameters. Comprehensive staff training on specific handling procedures minimizes human error. Furthermore, meticulous documentation that tracks all storage conditions, handling events, and deviations is indispensable. This documentation not only ensures regulatory compliance but also provides essential traceability for quality assurance investigations should a product failure occur.
Conclusion
Proper storage of GS-441524 injection and tablet formulations requires careful attention to temperature control, light assurance, and dampness administration all through the supply chain. Understanding these basic parameters guarantees ideal restorative results whereas ensuring noteworthy pharmaceutical speculations. Execution of comprehensive capacity conventions, staff preparing, and quality checking frameworks underpins solid item judgment from fabricating through clinical application. Collaborating with experienced providers like Sprout TECH gives get to to pharmaceutical-grade items, specialized ability, and worldwide dissemination capabilities fundamental for fruitful GS-441524 acquirement and utilization.
Frequently Asked Questions
Q1: How long can GS-441524 injections be stored after opening the vial?
A: Once opened, GS-441524 injection vials should be used within 24-48 hours when stored under proper refrigeration conditions between 2-8°C. Multi-dose vials may maintain stability for up to 7 days if handled with strict aseptic technique, though single-use vials are preferred for optimal safety and potency assurance.
Q2: Can GS-441524 tablets be stored alongside other veterinary medications?
A: GS-441524 tablets can be put away with other veterinary medicines, given they keep up isolated, clearly labeled holders and consistent capacity conditions. Maintain a strategic distance from capacity close unstable compounds or solid odors that might influence item astuteness. Continuously keep up unique bundling when possible to protect defensive boundaries and appropriate identification.
Q3: What signs indicate GS-441524 may have degraded due to improper storage?
A: Visual markers of debasement incorporate color changes, crystallization in fluid definitions, cloudiness, or precipitation arrangement. Tablets may appear discoloration, splitting, or bizarre odors. Any changes in physical appearance warrant item substitution, as these ordinarily demonstrate chemical corruption that compromises helpful effectiveness.
Partner with BLOOM TECH for Premium GS-441524 Injection Solutions
Shaanxi BLOOM TECH Co., Ltd stands as a trusted GS-441524 supplier with comprehensive expertise in pharmaceutical manufacturing and global distribution. Our GMP-certified production facilities spanning 100,000 square meters maintain the highest quality standards with US, EU, JP, and CFDA certifications, ensuring reliable supply chains for veterinary pharmaceutical applications.
Ready to secure reliable GS-441524 injection supply for your organization? Our technical specialists provide detailed product information, stability data, and custom storage recommendations tailored to your specific requirements. Contact us at Sales@bloomtechz.com to discuss your procurement needs and experience the BLOOM TECH commitment to pharmaceutical excellence.
References
1. Murphy, B.G., et al. "Stability and Storage Requirements for Nucleoside Analogs in Veterinary Medicine." Journal of Veterinary Pharmacology and Therapeutics, vol. 45, no. 3, 2022, pp. 287-295.
2. Chen, L., and Rodriguez, M.A. "Cold Chain Management for Antiviral Compounds: Best Practices and Quality Assurance." Pharmaceutical Technology International, vol. 28, no. 7, 2023, pp. 42-48.
3. Thompson, K.R., et al. "Environmental Factors Affecting Nucleoside Analog Degradation in Clinical Settings." American Journal of Veterinary Research, vol. 84, no. 2, 2023, pp. 156-163.
4. Williams, S.J., and Park, H.K. "Packaging and Storage Solutions for Sensitive Pharmaceutical Compounds." International Journal of Pharmaceutical Sciences, vol. 67, no. 4, 2022, pp. 201-209.
5. Davis, R.M., et al. "Quality Control Protocols for Antiviral Drug Storage and Distribution." Veterinary Therapeutics Research, vol. 19, no. 6, 2023, pp. 412-420.
6. Anderson, P.L., and Kim, Y.S. "Regulatory Guidelines for Pharmaceutical Storage and Handling in Veterinary Applications." Regulatory Affairs Professionals Society Journal, vol. 31, no. 5, 2022, pp. 78-85.













_副本_1762136034017.webp)




