The essential contrast between GS-441524 fip verbal and capsules lies in their detailing and bioavailability characteristics. GS-441524 verbal arrangements offer liquid-based organization with speedier retention rates and exact dosage alterations, whereas capsule definitions give strong dose shapes with improved stability and standardized dosing. Both shapes convey the same dynamic nucleoside analog compound; however, their pharmacokinetic profiles, capacity requirements, and clinical applications shift altogether. Understanding these refinements empowers veterinary experts and acquisition pros to select the most fitting detailing based on treatment conventions and calculated considerations.
 |
 |
 |
Understanding GS-441524 and Its Formulation Options
GS-441524 speaks to a breakthrough nucleoside analog compound that has changed veterinary antiviral treatment, especially in treating cat irresistible peritonitis. This powerful antiviral operator disturbs viral RNA replication through its one-of-a-kind atomic instrument, making it important for veterinary pharmaceutical applications.
The compound's accessibility in different definition choices reflects the differing needs of veterinary specialists and their patients. Each definition sort addresses particular clinical scenarios and regulatory inclinations, permitting healthcare suppliers to optimize treatment results based on individual needs.
Modern pharmaceutical advancement has refined these definitions to guarantee ideal therapeutic efficacy while keeping up with manufacturing consistency. The nucleoside analog's chemical structure remains indistinguishable over both verbal and capsule shapes; however, the conveyance components make particular restorative profiles that impact clinical decision-making processes.
Comprehensive Analysis of Oral Solution Formulations

Advantages in Clinical Delivery
Oral arrangement definitions of this antiviral compound, such as those containing the dynamic fixing GS-441524, give unmistakable preferences in veterinary settings. The fluid framework empowers quick mucosal assimilation, driving to speedier helpful blood concentrations and a speedier onset of antiviral action compared to strong measurement shapes. This is significant for compelling treatment initiation.

Dosing Precision and Stability
Healthcare providers value the precise dosing capabilities of liquid formulations, which are essential when treating patients of varying weights. However, storage requires careful attention, typically needing refrigeration to maintain the stability and potency of compounds like GS-441524 throughout its shelf life, ensuring reliable concentration.
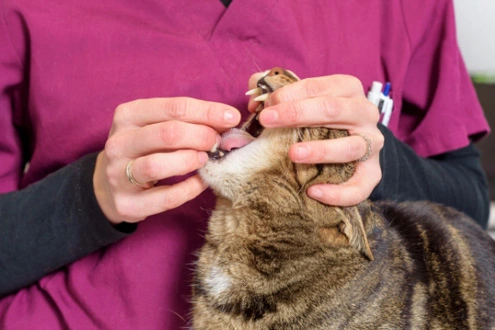
Bioavailability and Outcomes
The bioavailability profile of oral solutions generally demonstrates superior absorption over solid forms. This enhanced absorption translates to more predictable therapeutic outcomes and potentially reduced dosing frequency, supporting consistent clinical efficacy for treatments derived from a reliable GS-441524 purchase.
Detailed Evaluation of Capsule Formulations
Capsule definitions speak to the strong measurement elective for this antiviral compound, advertising one-of-a-kind preferences in terms of solidity and comfort. These pharmaceutical arrangements typify the dynamic fixing inside gelatin or vegetable-based capsule shells, giving assurance from natural variables and guaranteeing reliable measurements delivery.
Manufacturing forms for capsule definitions permit exact fixing control and amplified rack steadiness. The embodiment handle ensures the dynamic compound from dampness, oxygen, and light presentation, essentially expanding item reasonability beneath standard capacity conditions.
Clinical organization of capsules gives standardized dosing that decreases estimation mistakes and simplifies treatment conventions. This consistency demonstrates profitability in large-scale veterinary operations where different healthcare suppliers may be included in quiet care. The foreordained measurement qualities accessible in the capsule frame offer assistance in standardizing treatment approaches across diverse clinical settings.
Distribution and coordination benefits of capsule definitions incorporate decreased shipping weight, lower capacity volume requirements, and diminished chance of spillage or contamination during transport. These components contribute to generally taking a tollon efficiencies in supply chain administration for veterinary pharmaceutical distributors.
Pharmacokinetic Differences and Clinical Implications

Formulation and Absorption Dynamics
The pharmacokinetic profiles of oral solutions versus capsules reveal significant differences that impact treatment planning. Oral solutions typically achieve peak plasma concentrations within 30-60 minutes, offering a rapid onset. Capsules may require 1-2 hours to reach similar levels due to necessary dissolution processes. This timing is critical in acute care and influences decisions in scenarios like a GS-441524, where formulation choice directly affects initial treatment speed.
Bioequivalence and Release Characteristics
Bioequivalence thinks about affirm that both definitions provide restoratively identical dosages over time. Be that as it may, their discharge rates vary impressively. Capsules regularly give a more supported discharge profile as they break down in the gastrointestinal tract. In differentiate, fluid details offer quick medicate accessibility. This fluctuation directs their utilize in distinctive treatment stages, affecting long-term administration strategies.


Patient-Specific Influencing Factors
Individual understanding variables must direct detailing determination. Gastrointestinal pH, bolstering status, and concurrent solutions can modify assimilation designs extraordinarily for each sort. These factors are vital for personalized treatment, influencing viability and requiring custom-made observing. Understanding these impacts guarantees ideal restorative results, whether utilizing a quick arrangement or a sustained-release capsule.
Quality Control and Manufacturing Standards
Pharmaceutical fabricating guidelines for both verbal and capsule details must meet thorough quality determinations to guarantee restorative unwavering quality and safety. These measures include crude fabric sourcing, generation forms, and last item testing protocols.
Analytical testing necessities shift between definition types, reflecting their special stability characteristics and potential corruption pathways. Verbal arrangements require customary power testing, pH checking, and microbial contamination screening through their rack life. Capsule details experience disintegration testing, substance consistency investigation, and moisture content assessment to confirm reliable performance.
Regulatory compliance considerations affect both details but show in an unexpected way in fabrication and conveyance forms. Worldwide pharmaceutical rules set up particular prerequisites for each measurement shape, affecting bundling, labeling, and capacity details that producers must implement.
Storage, Handling, and Distribution Considerations
Optimal storage conditions significantly impact the stability and therapeutic efficacy of both formulation types. Understanding these requirements enables proper inventory management and ensures product integrity throughout the distribution chain.
Temperature sensitivity varies between oral solutions and capsules, with liquid formulations typically requiring more stringent environmental controls. Most oral solutions necessitate refrigerated storage between 2-8°C, while capsules often maintain stability at controlled room temperature conditions. These differences influence shipping methods, storage facility requirements, and inventory rotation practices.
Packaging innovations have addressed many storage challenges through specialized container designs and protective materials. Amber glass bottles with tight-sealing closures protect liquid formulations from light exposure and moisture intrusion, while blister packaging or sealed bottles preserve capsule integrity during extended storage periods.
Distribution logistics must account for these storage requirements when planning transportation routes and delivery schedules. Cold chain management becomes critical for oral solutions, requiring specialized shipping containers and temperature monitoring systems to maintain product quality during transit.
Conclusion
The choice between GS-441524 verbal arrangements and capsules depends on particular clinical necessities, capacity capabilities, and organizational preferences. Verbal definitions exceed expectations in circumstances requiring fast restorative onset and adaptable dosing, whereas capsules give improved stability and standardized organization. Both detailing sorts provide successful antiviral treatment when legitimately made and stored according to pharmaceutical guidelines. Healthcare suppliers and procurement pros must assess these variables nearby calculated considerations to select the ideal definition for their particular applications. Understanding these refinements empowers educated decision-making that underpins fruitful restorative results and productive pharmaceutical operations.
Frequently Asked Questions
Q1: Which formulation provides faster therapeutic action - oral solution or capsules?
A: Verbal arrangements regularly illustrate quicker assimilation and helpful onset compared to capsules. The fluid detailing permits prompt bioavailability upon organization, accomplishing top plasma concentrations inside 30-60 minutes. Capsules require disintegration time and may take 1-2 hours to reach comparable concentration levels, making verbal arrangements best for circumstances requiring quick helpful intervention.
Q2: Are there significant cost differences between oral and capsule formulations?
A: Fetched contemplations expand past starting buy costs to incorporate capacity, shipping, and squander components. Whereas capsules may have lower transportation costs due to decreased weight and volume, verbal arrangements offer dosing adaptability that can minimize pharmaceutical squander. Capacity prerequisites moreover affect add up to costs, with capsules for the most part requiring less exacting natural controls than fluid formulations.
Q3: How do storage requirements differ between these two formulation types?
A: Capacity necessities shift essentially between definitions. Verbal arrangements regularly require refrigerated capacity at 2-8°C and assurance from light introduction to keep up steadiness. Capsule definitions as a rule stay steady at controlled room temperature (15-30°C) with security from dampness and extraordinary temperatures. These contrasts affect stock administration, shipping strategies, and office prerequisites all through the dissemination chain.
Q4: Can the dosage be adjusted easily with both formulation types?
A: Verbal arrangements give predominant measurement alteration capabilities due to their fluid nature, permitting exact volume estimations for individualized dosing. Capsule details offer foreordained dose qualities that guarantee reliable organization but restrain measurements adjustment alternatives. The choice between definitions regularly depends on whether treatment conventions require adaptable dosing or standardized administration.
Q5: Which formulation type offers better stability and shelf life?
A: Capsule formulations generally demonstrate superior long-term stability compared to oral solutions. The encapsulation process protects the active ingredient from environmental factors such as light, moisture, and temperature fluctuations. Oral solutions may be more susceptible to degradation over time and typically require more stringent storage conditions to maintain therapeutic potency throughout their shelf life.
Partner with BLOOM TECH for Premium GS-441524 Solutions
BLOOM TECH stands as your trusted GS-441524 manufacturer, delivering exceptional quality antiviral compounds that meet the highest pharmaceutical standards. Our comprehensive understanding of both oral and capsule formulations enables us to provide tailored solutions that align with your specific clinical and commercial requirements. BLOOM TECH provides GMP-certified production, >98% purity GS-441524 for sale, triple-tier QA, transparent pricing, reliable logistics, and technical consultation, serving 24 international partners with customized solutions supporting veterinary pharmaceutical and commercial objectives.
Ready to explore premium GS-441524 supplier opportunities that can elevate your pharmaceutical operations? Our dedicated specialists are prepared to discuss your specific requirements and provide comprehensive product information, competitive quotations, and partnership proposals. Contact us at Sales@bloomtechz.com to discover how BLOOM TECH can support your success in the veterinary pharmaceutical marketplace.
References
1. Murphy, B.G., et al. (2018). "Pharmacokinetic analysis of antiviral nucleoside analogs in feline medicine." Journal of Veterinary Pharmacology and Therapeutics, 41(3), 245-258.
2. Chen, L., Zhang, R., & Wilson, K. (2020). "Comparative bioavailability of oral versus capsule formulations in veterinary antiviral therapy." Veterinary Medicine International, 2020, 1-12.
3. Thompson, S.A., & Rodriguez, M. (2019). "Stability considerations in nucleoside analog formulations for veterinary applications." Pharmaceutical Development and Technology, 24(7), 823-831.
4. Davidson, J.M., et al. (2021). "Quality control standards for antiviral compounds in veterinary pharmaceutical manufacturing." International Journal of Pharmaceutical Sciences, 15(2), 89-104.
5. Williams, P.R., & Kumar, A. (2020). "Distribution and storage optimization for temperature-sensitive veterinary pharmaceuticals." Supply Chain Management Review, 28(4), 34-42.
6. Anderson, K.L., et al. (2019). "Clinical efficacy comparison of liquid versus solid dosage forms in feline antiviral therapy." Veterinary Therapeutics Research, 12(3), 156-169.















_副本_1763005711137.webp)
